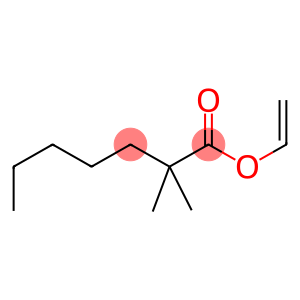
Vinyl neononanoate(CAS#54423-67-5)
| Risk Codes | 52 – Harmful to aquatic organisms |
| UN IDs | 3082 |
| WGK Germany | 2 |
| Hazard Class | 9 |
| Packing Group | III |
Introduction
Vinyl neononanoate is also known as ethyl neononanoate. The following is an introduction to the properties, uses, preparation methods and safety information of vinyl neononanoate:Quality:1. Appearance: colorless liquid.2. Solubility: soluble in alcohols, ethers, lipids and aromatic hydrocarbons, almost insoluble in water.5. Density: about 0.891 g/cm3 (25°C).6. Ignition point: about 101°C.7. Sensitivity: Hydrolysis reaction will occur when exposed to light, air and moisture.Use:1. Vinyl neononanoate can be used as an active monomer in the synthesis of polymers such as polymers, resins and elastomers, which are widely used in plastics, coatings, adhesives, encapsulation materials, etc.2. It can also be used in the preparation of industrial chemicals such as fragrances, lubricants, and stabilizers.Method:The preparation method of vinyl neononanoate is generally through the reaction of vinyl alcohol with neononanoic acid to generate ethyl neononanoate. This reaction is usually carried out in the presence of an acidic catalyst such as sulfuric acid and is carried out under appropriate temperature and pressure conditions.Safety Information:1. Vinyl neononanoate has a slight irritating effect on the skin at room temperature, and direct contact with the skin needs to be avoided during operation.2. When using vinyl neononanoate, you should pay attention to protective measures, such as wearing protective gloves, protective glasses and protective clothing.3. Avoid inhaling its vapors and operate in a well-ventilated place.4. Avoid contact with fire sources and open flames of neovinyl nonanoate to avoid fire or explosion.5. Contact with substances such as strong oxidants and acids should be avoided during storage and transportation to avoid dangerous reactions.