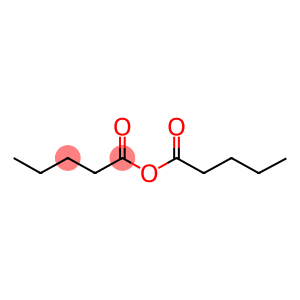
Valeric anhydride(CAS#2082-59-9)
| Hazard Symbols | C – Corrosive |
| Risk Codes | 34 – Causes burns |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) |
| UN IDs | UN 3265 8/PG 3 |
| WGK Germany | 3 |
| HS Code | 29159000 |
| Hazard Class | 8 |
| Packing Group | III |
Introduction
Valeric anhydride is an organic compound. The following is an introduction to the properties, uses, preparation methods and safety information of valeric anhydride:
Quality:
- Valeric anhydride is a colorless, transparent liquid with a pungent odor.
- It reacts with water to produce a mixture of valeric acid and valeric anhydride.
Use:
- Valeric anhydride is mainly used as a reagent and intermediate in organic synthesis.
- It can be used to prepare compounds with different functional groups, such as ethyl acetate, anhydrides, and amides.
- Valeric anhydride can also be used in the synthesis of pesticides and fragrances.
Method:
- Valeric anhydride is usually produced by the reaction of valeric acid with an anhydride (e.g. acetic anhydride).
- The reaction conditions can be carried out at room temperature or heated under the protection of inert gas.
Safety Information:
- Valeric anhydride is irritating and corrosive, avoid contact with skin and eyes, and make sure to operate in a well-ventilated area.
- During handling and storage, avoid contact with oxidants or strong acids and bases to avoid dangerous reactions.
- Follow safe handling protocols for chemicals and equip yourself with appropriate protective equipment such as lab gloves, safety glasses, etc.