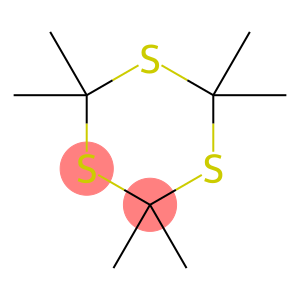
Trithioacetone(CAS#828-26-2)
| Risk Codes | R36/37/38 – Irritating to eyes, respiratory system and skin. R36/38 – Irritating to eyes and skin. R11 – Highly Flammable |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S37/39 – Wear suitable gloves and eye/face protection S9 – Keep container in a well-ventilated place. S33 – Take precautionary measures against static discharges. S16 – Keep away from sources of ignition. |
| UN IDs | UN 3334 |
| WGK Germany | 2 |
| RTECS | YL8350000 |
| HS Code | 29309090 |
Introduction
Trithioacetone, also known as ethylenedithione. The following is an introduction to the properties, uses, preparation methods and safety information of trithiacetone:
Quality:
- Appearance: Trithiacetone is a colorless to yellowish liquid.
- Odor: Has a strong sulfur taste.
- Solubility: Soluble in organic solvents such as ethanol, ethers and ketones.
Use:
- Trithiacetone is commonly used in organic synthesis as a vulcanizing agent, reducing agent and coupling reagent.
- It is used in the preparation of organic sulfides, such as various sulfur-containing heterocyclic compounds.
- In the rubber industry, it can be used as a accelerator.
- Can also be used as an additive for metal cleaning and electroplating solutions.
Method:
- Trithioneone can be obtained by reacting iodoacetone with sulfur in the presence of carbon disulfide (CS2) and dimethyl sulfoxide (DMSO).
- Reaction equation: 2CH3COCI + 3S → (CH3COS)2S3 + 2HCI
Safety Information:
- Trithiacetone has a pungent odor and should avoid inhaling high concentrations of gases.
- When in contact with the skin, it may cause irritation, irritation, or skin damage.
- Wear appropriate protective gear, including protective eyewear and gloves, when in use.
- Avoid contact with fire sources and strong oxidants during storage, and keep it well ventilated.