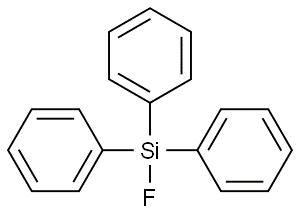
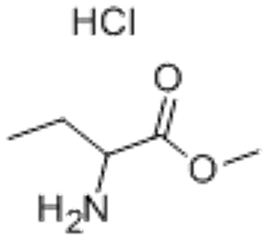Triphenylfluorosilane(CAS# 379-50-0)
Introduction
It is insoluble in water at room temperature, but it can be dissolved in some organic solvents such as benzene and methylene chloride. It has good hydrophobicity and chemical stability, and can resist the attack of acids, alkalis and oxidants to a certain extent.
In practical applications, triphenylmethylfluorosilane is often used as a reagent in organic synthesis. It can be used to introduce silicone groups and change the chemical properties of molecules. It can also be used as a catalyst for organometallic chemical reactions. Triphenylmethylfluorosilane can also be used as a surface modifier to improve the properties of certain materials.
The preparation method of triphenylmethylfluorosilane is generally obtained by the reaction of triphenylmethyllithium and magnesium silicon fluoride. Magnesium silicon fluoride is suspended in anhydrous ether and then tritylmethyllithium is slowly added. The reaction needs to be kept low to avoid side reactions. After the reaction is complete, the pure triphenylmethylfluorosilane is separated from the reaction mixture by a common organic reaction step.
When using triphenylmethylfluorosilane, the following safety precautions should be observed: it is a flammable liquid and may cause a fire if it encounters an ignition source. It should be stored in a cool, well-ventilated place, away from fire and oxidants. Appropriate personal protective equipment such as protective eyewear and gloves need to be worn during operation. Avoid direct contact with the skin and inhalation of its vapors.