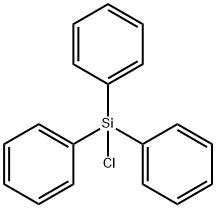
Triphenylchlorosilane; P3;TPCS (CAS#76-86-8)
| Hazard Symbols | C – Corrosive |
| Risk Codes | R34 – Causes burns R37 – Irritating to the respiratory system |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S24/25 – Avoid contact with skin and eyes. |
| UN IDs | UN 3261 8/PG 2 |
| WGK Germany | 1 |
| RTECS | VV2720000 |
| FLUKA BRAND F CODES | 10-21 |
| TSCA | Yes |
| HS Code | 29310095 |
| Hazard Class | 8 |
| Packing Group | II |
Introduction
Triphenylchlorosilane. Its properties are as follows:
1. Appearance: colorless liquid, volatile at room temperature.
4. Density: 1.193 g/cm³.
5. Solubility: soluble in non-polar solvents, such as ether and cyclohexane, react with water to form silicic acid.
6. Stability: Stable under dry conditions, but will react with water, acids and alkalis.
The main uses of triphenylchlorosilanes:
1. As a reagent in organic synthesis: it can be used as a silicon source in organic reactions, such as silene synthesis, organometallic catalytic reaction, etc.
2. As a protective agent: triphenylchlorosilane can protect hydroxyl and alcohol-related functional groups, and is often used as a reagent to protect alcohols and hydroxyl groups in organic synthesis.
3. As a catalyst: Triphenylchlorosilane can be used as a ligand for certain transition metal-catalyzed reactions.
The preparation method of triphenylchlorosilane is generally obtained by the chlorination reaction of triphenylmethyltin, and the specific steps can be referred to the relevant organic synthesis literature.
1. Triphenylchlorosilane is irritating to the eyes and skin, so avoid contact with it.
2. Pay attention to protective measures when using, and wear appropriate protective glasses and gloves.
3. Avoid inhaling its vapors and operate in a well-ventilated area.
4. When handling triphenylchlorosilanes, avoid contact with water, acids, and alkalis to avoid dangerous gases or chemical reactions.
5. When storing and using, it should be properly sealed and stored, away from fire sources and high temperatures.
The above is the nature, use, preparation method and safety information of triphenylchlorosilane. If necessary, exercise caution and follow the relevant laboratory safety practices.