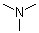Trimethylamine(CAS#75-50-3)
| Risk Codes | R36/37/38 – Irritating to eyes, respiratory system and skin. R34 – Causes burns R20/22 – Harmful by inhalation and if swallowed. R12 – Extremely Flammable R41 – Risk of serious damage to eyes R37/38 – Irritating to respiratory system and skin. R20 – Harmful by inhalation R11 – Highly Flammable |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S16 – Keep away from sources of ignition. S29 – Do not empty into drains. S39 – Wear eye / face protection. S3 – Keep in a cool place. |
| UN IDs | UN 2924 3/PG 2 |
| WGK Germany | 1 |
| RTECS | YH2700000 |
| FLUKA BRAND F CODES | 3-10 |
| TSCA | Yes |
| HS Code | 29211100 |
| Hazard Class | 3 |
| Packing Group | II |
Introduction
Trimethylamine is a type of organic compound. It is a colorless gas with a strong pungent odor. The following is an introduction to the properties, uses, preparation methods and safety information of trimethylamine:
Quality:
Physical properties: Trimethylamine is a colorless gas, soluble in water and organic solvents, and forms a flammable mixture with air.
Chemical Properties: Trimethylamine is a nitrogen-carbon hybrid, which is also an alkaline substance. It can react with acids to form salts, and can react with some carbonyl compounds to form corresponding amination products.
Use:
Organic synthesis: Trimethylamine is often used as an alkali catalyst in organic synthesis reactions. It can be used in the preparation of organic synthesis reactions such as esters, amides, and amine compounds.
Method:
Trimethylamine can be obtained by the reaction of chloroform with ammonia in the presence of an alkali catalyst. The specific preparation method can be:
CH3Cl + NH3 + NaOH → (CH3)3N + NaCl + H2O
Safety Information:
Trimethylamine has a pungent odor and exposure to high concentrations of trimethylamine can cause eye and respiratory irritation.
Because trimethylamine is less toxic, it generally has no obvious harm to the human body under reasonable use and storage conditions.
Trimethylamine is a flammable gas, and its mixture has a risk of explosion at high temperatures or open flames, and should be stored in a cool, well-ventilated place to avoid contact with open flames and high temperatures.
Contact with oxidants, acids or other combustibles should be avoided during operation to avoid dangerous reactions.