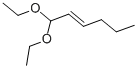
trans-2-Hexen-1-Al Diethyl Acetal(CAS#54306-00-2)
| Hazard Symbols | Xi – Irritant |
| Risk Codes | 36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. |
| WGK Germany | 3 |
trans-2-Hexen-1-Al Diethyl Acetal(CAS#54306-00-2) introduce
physical property
Appearance: It usually appears as a colorless to light yellow transparent liquid, which makes it more convenient to operate in chemical production processes such as material transportation and mixing reactions.
Smell: It has a unique fruity smell, which is fresh and natural. This feature has attracted much attention in the field of fragrance essence, and can be used as the key raw material for blending fruity flavor.
Solubility: It can dissolve well in most organic solvents, such as ethanol, ether, acetone, etc., making it easy to mix and contact with other reactants in organic synthesis reaction systems; The solubility in water is relatively limited, which conforms to the dissolution law of organic compounds with high carbon content.
Boiling point: It has a specific boiling point range, which is an important basis for separation and purification operations such as distillation and rectification. The boiling point of samples with different purities may vary slightly, and the quality and purity of the product can be preliminarily evaluated by accurately measuring the boiling point.
4、 Chemical properties
Acetal hydrolysis reaction: Under acidic conditions, the diethylacetal structure in the molecule is prone to hydrolysis, generating aldehyde groups and ethanol again. This characteristic is often utilized in organic synthesis for functional group conversion or aldehyde group protection, and is released at the appropriate time to participate in subsequent reactions.
Double bond addition reaction: Carbon carbon double bonds can act as active sites and undergo addition reactions with hydrogen, halogens, etc. By controlling the reaction conditions and reagent dosage, a series of derivatives can be selectively prepared, enriching the diversity of compounds.
Oxidation reaction: Under the action of appropriate oxidants, molecules can undergo oxidation, double bond breakage, or further oxidation of aldehyde groups to generate corresponding oxidation products, providing a pathway for the synthesis of other complex compounds.
5、 Synthesis method
The common synthetic pathway is to start with trans-2-hexenal and react it with anhydrous ethanol in the presence of acidic catalysts such as dry hydrogen chloride gas, p-toluenesulfonic acid, etc. The reaction process requires strict temperature control, usually in the range of low temperature to room temperature, to prevent side reactions from occurring; At the same time, it is necessary to ensure an anhydrous environment, as the presence of water can reverse the aldol reaction and affect the yield. After the reaction is completed, the catalyst is usually neutralized with alkaline solution, and then separated by distillation, rectification and other methods to obtain high-purity target products.