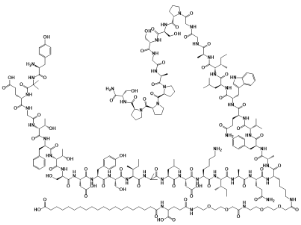
Tirzepatide(CAS#2023788-19-2)
Introduction
Tirpatide is the world’s first and currently the only GIP/GLP-1 receptor agonist.Eli Lilly’s tirzepatide was successful in a Phase 3 study showing benefit in adults with heart failure who maintain ejection fraction and obese. On May 21, 2024, according to the official website of the State Food and Drug Administration, Eli Lilly’s dual-target blockbuster hypoglycemic drug tirpatide was approved for marketing for adult patients with type 2 diabetes who are still poorly controlled with metformin and/or sulfonylureas on the basis of diet control and exercise.INNAPOLIS, Aug. 1, 2024 /PRNewswire/ — Eli Lilly & Company (NYSE:LLY) announced positive topline results from the SUMMIT Phase 3 clinical trial, which evaluated the safety and efficacy of tirzepatide injection (5 mg, 10 mg, or 15 mg) in adults with heart failure, preservation of ejection fraction (HFpEF), and obesity. Compared to placebo, Tirzepatide showed statistically significant improvements in both primary endpoints, a reduction in the risk of heart failure outcomes (assessed as a composite endpoint), and an improvement in heart failure symptoms and physical limitations as measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ) Clinical Summary Score (CSS).All key secondary endpoints were also met, including improvement in exercise capacity as measured by 6-minute walk test distance (6MWD), reduction in the inflammatory marker high-sensitivity C-reactive protein (hsCRP), and a decrease in mean body weight from baseline at 52 weeks. For efficacy estimates, tezepatide ii resulted in a weight loss of 15.7% compared to placebo (2.2%). For the treatment regimen estimate, tezepatide iii resulted in a 13.9% weight loss compared to 2.2% for placebo.Jeff Emmick, Ph.D., senior vice president of product development at Eli Lilly & Company, said, “HFpEF accounts for nearly half of all heart failure cases, and nearly 60% of affected people in the U.S. also suffer from obesity. 1,2 Although the number of people with HFpEF and obesity continues to increase, treatment options remain limited. ”。“ Previous incretin studies in this population have focused on symptoms and physical limitations. In a first-of-its-kind trial, tirzepatide reduced symptom severity and improved prognosis for heart failure in patients with HFpEF and obesity. ”HFpEF is a condition in which the left pumping chamber of the heart becomes stiff and does not fill properly. It is associated with a high burden of symptoms and physical limitations that affect daily life, including fatigue, shortness of breath, decreased exercise capacity, and swelling of the limbs.The overall safety profile of tirzepatide in the SUMMIT trial was consistent with previously reported tirzepatin studies, including SURMOUNT and SURPASS. The most common adverse events reported at SUMMIT were predominantly gastrointestinal adverse events and were typically mild to moderate in severity. The most common adverse events in patients treated with tirzepatide were diarrhea, nausea, constipation, and vomiting.Eli Lilly & Company will continue to evaluate the results of the SUMMIT, which will be presented at an upcoming medical conference