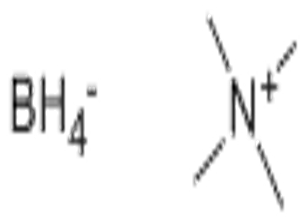Tetramethylammonium borohydride(CAS# 16883-45-7)
Risk and Safety
| Risk Codes | R15 – Contact with water liberates extremely flammable gases R25 – Toxic if swallowed R36/38 – Irritating to eyes and skin. R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S43 – In case of fire use … (there follows the type of fire-fighting equipment to be used.) S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36 – Wear suitable protective clothing. |
| UN IDs | UN 3134 4.3/PG 2 |
| WGK Germany | 3 |
| RTECS | BS8310000 |
| TSCA | Yes |
| Hazard Class | 4.3 |
Tetramethylammonium borohydride(CAS# 16883-45-7) introduction
Tetramethylammonium borohydride is a common organoboron compound. The following is an introduction to its nature, use, manufacturing methods and safety information:
Quality:
Tetramethylammonium borohydride is a colorless crystalline solid that is easily soluble in water. It is a weakly alkaline substance that reacts with acids to form corresponding salts. It is sensitive to light and heat and should be kept in a cool, dry place.
Use:
Tetramethylammonium borohydride is commonly used as a catalyst in chemical reactions in organic synthesis. It can be used in the synthesis of organoboron compounds, boranes, and other compounds. In addition, it can also be used as a reducing agent for the reduction of metal ions or organic compounds, and can be used to synthesize metal-organic compounds.
Method:
The preparation of tetramethylboroammonium hydride usually uses the reaction of methyllithium and trimethylborane. Lithium methyl and trimethylborane react at low temperatures to form lithium methylborohydride. Then, lithium methylborohydride is reacted with methylammonium chloride to obtain tetramethylammonium borohydride.
Safety Information:
Tetramethylammonium borohydride is relatively safe under normal conditions of use. Care should be taken to avoid contact with skin, eyes or mouth when carrying or handling. It should be kept away from fire sources and combustible substances and stored in an airtight container.