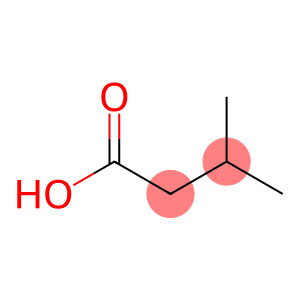
sovalericacid(CAS#503-74-2)
| Risk Codes | R34 – Causes burns R24 – Toxic in contact with skin R22 – Harmful if swallowed |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S38 – In case of insufficient ventilation, wear suitable respiratory equipment. S28A - |
| UN IDs | UN 3265 8/PG 2 |
| WGK Germany | 1 |
| RTECS | NY1400000 |
| FLUKA BRAND F CODES | 13 |
| TSCA | Yes |
| HS Code | 2915 60 90 |
| Hazard Class | 6.1 |
| Packing Group | III |
| Toxicity | LD50 i.v. in mice: 1120±30 mg/kg (Or, Wretlind) |
Introduction
Isovaleric acid. The following is an introduction to the properties, uses, preparation methods and safety information of isovaleric acid:
Quality:
Appearance: Colorless or yellowish liquid with a pungent odor similar to acetic acid.
Density: 0.94g/cm³
Solubility: soluble in water, can also be miscible with ethanol, ether and other organic solvents.
Use:
Synthesis: Isovaleric acid is an important chemical synthesis intermediate, which is widely used in many industrial fields such as organic synthesis, pharmaceuticals, coatings, rubber and plastics.
Method:
The preparation method of isovaleric acid includes the following ways:
Through the oxidation reaction of n-butanol, the oxidation of n-butanol to isovaleric acid is carried out using an acidic catalyst and oxygen.
Magnesium butyrate is formed by the reaction of magnesium butyl bromide with carbon dioxide, which is then converted to isovaleric acid by reaction with carbon monoxide.
Safety Information:
Isovaleric acid is a corrosive substance, avoid contact with skin and eyes, and pay attention to the use of protective gloves, safety glasses and protective clothing.
When using isovaleric acid, inhalation of its vapours should be avoided and the operation should be carried out in a well-ventilated environment.
The ignition point is low, avoid contact with the fire source, and store away from open flames and heat sources.
In case of accidental exposure to isovaleric acid, rinse immediately with plenty of water and seek medical attention.