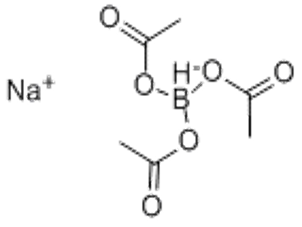Sodium triacetoxyborohydride(CAS# 56553-60-7)
| Risk Codes | R15 – Contact with water liberates extremely flammable gases R34 – Causes burns R14/15 - R37/38 – Irritating to respiratory system and skin. R11 – Highly Flammable |
| Safety Description | S43 – In case of fire use … (there follows the type of fire-fighting equipment to be used.) S7/8 - S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| UN IDs | UN 1409 4.3/PG 2 |
| WGK Germany | 3 |
| FLUKA BRAND F CODES | 10-21 |
| TSCA | Yes |
| HS Code | 29319090 |
| Hazard Note | Irritant/Flammable |
| Hazard Class | 4.3 |
| Packing Group | III |
Introduction
Sodium triacetoxyborohydride is an organoboron compound with the chemical formula C6H10BNaO6. The following is an introduction to its properties, uses, manufacturing methods and safety information:
Quality:
1. Appearance: Sodium triacetoxyborohydride is usually a colorless crystalline solid.
2. Stability: It is relatively stable at room temperature and can be dissolved in many organic solvents.
3. Toxicity: Sodium triacetoxyborohydride is less toxic compared to other boron compounds.
Use:
1. Reducing agent: Sodium triacetoxyborohydride is a commonly used reducing agent for organic synthesis, which can effectively reduce aldehydes, ketones and other compounds to the corresponding alcohols.
2. Catalyst: Sodium triacetoxyborohydride can be used as a catalyst in some organic synthesis reactions, such as Bar-Fischer ester synthesis and Swiss-Haussmann reaction.
Method:
The preparation method of triacetoxyborohydride is generally obtained by the reaction of triacetoxyborohydride with sodium hydroxide. For the specific process, please refer to the handbook of organic chemical synthesis and other relevant literature.
Safety Information:
1. Sodium triacetoxyborohydride is irritating to the skin and eyes, so care should be taken to avoid contact during operation, and wear protective gloves and goggles if necessary.
2. When storing and handling, avoid contact with water vapor in the air as it is sensitive to water and will decompose.
Given the special nature of chemicals, please use and handle them under the guidance of a professional.