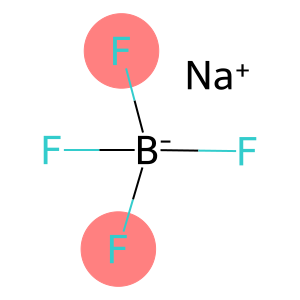
sodium tetrafluoroborate(CAS#13755-29-8)
| Risk Codes | 34 – Causes burns |
| Safety Description | S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S25 – Avoid contact with eyes. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| UN IDs | 3260 |
| WGK Germany | 1 |
| RTECS | ED2975000 |
| FLUKA BRAND F CODES | 21 |
| TSCA | Yes |
| HS Code | 2826 90 80 |
| Hazard Note | Irritant |
Introduction
Sodium fluoroborate.Quality:2. It is soluble in water and some polar organic solvents, but insoluble in alcohols.3. It is highly corrosive, and acid mineral reactions will occur when encountering acids.Use:1. Sodium fluoroborate is often used as a catalyst and reducing agent in organic synthesis, especially in hydrogenation reactions.2. It is also used as a solvent and electrolyte for platinum metal in the preparation of optical and electronic devices.3. Sodium fluoroborate can also be used as an additive for electroplating, a glass etching agent and a component of erosion solutions.Method:Sodium fluoroborate can be prepared by the reaction of hydrofluoric acid and sodium borate, which produces HBF4 and NaHF2 during the reaction, and then goes through crystallization, drying and other steps to obtain pure products.Safety Information:1. Sodium fluoroborate is irritating and corrosive, and should be avoided in contact with the skin and eyes, and avoid inhaling its dust or vapor.2. Wear appropriate protective gloves, glasses, and a protective face shield when using.4. When storing and transporting sodium fluoroborate, it should be kept away from acids and combustibles, stored in a sealed container, and avoid contact with moisture.