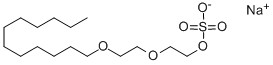
Sodium Laureth Sulfate CAS 3088-31-1
Sodium Laureth Sulfate CAS 3088-31-1 Information
Physical
Appearance: The common sodium laureth sulfate is a colorless or light yellow viscous liquid, this viscous texture stems from intermolecular interactions, such as hydrogen bonding, which also determines that it needs to be adapted to specific equipment in packaging and transportation to prevent residues and clogging.
Solubility: It has excellent water solubility, thanks to the polyether chain segment and sulfonic acid group in the molecular structure, which can be rapidly ionized in water to form a stable anion, which makes the whole molecule easily dispersed in water to form a clear and transparent solution, which is convenient for application in various water-based formula systems.
Melting point and density: Since it is a liquid, it is of little significance to talk about melting point; Its density is generally slightly higher than that of water, between 1.05 and 1.08 g/cm³, and the density data helps to accurately calculate the volume and mass conversion during formulation and dosing.
Chemical properties
Surfactant: As a potent surfactant, it significantly reduces the surface tension of water. When added to water, the molecules will spontaneously migrate to the air-water interface, with the hydrophobic end reaching towards the air and the hydrophilic end remaining in the water, disrupting the originally tight arrangement of water molecules, making it easier for water to spread and wet on solid surfaces, thereby enhancing the ability to clean, emulsify, foam, etc.
Stability: It can maintain good chemical stability in a wide pH range (usually pH 4 – 10), which makes it suitable for a variety of product formulations in different acid-alkali environments, but under the long-term action of strong acids and alkalis, hydrolysis and decomposition may also occur, affecting performance.
Interaction with other substances: when it encounters cationic surfactants, it will form a precipitate due to charge attraction and lose its surface activity; However, when combined with other anionic and nonionic surfactants, it can often synergize to further optimize the cleaning and foaming performance of the formulation.
Preparation method:
Generally, lauryl alcohol is used as the starting material, and the ethoxylation reaction is carried out first, and different numbers of ethylene oxide units are introduced to obtain laureth. Subsequently, after sulfonation and neutralization steps, the laureth polyester is treated with sulfonating agents such as sulfur trioxide, and then neutralized by adding sodium hydroxide to finally prepare sodium laureth sulfate. The whole process is strictly controlled by reaction temperature, pressure and material ratio, and the product quality will be affected if there is a slight difference in the pool.
use
Personal care products: It is a key ingredient in cleaning products such as shampoos, shower gels, and hand sanitizers, which are responsible for producing a rich and dense lather for a pleasant use experience, while powerfully removing oil and dirt from the skin and hair, leaving users feeling refreshed and clean.
Household cleaners: In household cleaning products such as dish soap and laundry detergent, SLES’s high cleaning power and good water solubility help to effectively remove stubborn stains on dishes and clothes, and its foaming properties can also assist users to judge the degree of cleanliness.
Industrial cleaning: In some industrial scenarios, such as metal cleaning and car cleaning, it also helps remove impurities such as oil and dust and improve cleaning efficiency and quality with its outstanding decontamination and emulsification capabilities.