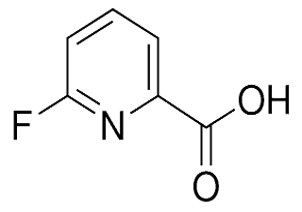
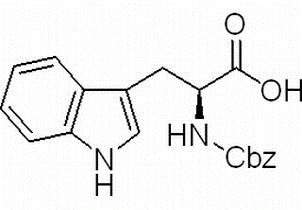
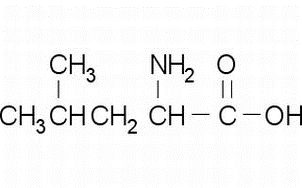
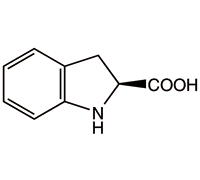
(S)-Indoline-2-carboxylic Acid(CAS# 79815-20-6)
| Hazard Symbols | Xn – Harmful |
| Risk Codes | R43 – May cause sensitization by skin contact R48/22 – Harmful danger of serious damage to health by prolonged exposure if swallowed. R62 – Possible risk of impaired fertility |
| Safety Description | S22 – Do not breathe dust. S25 – Avoid contact with eyes. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37 – Wear suitable protective clothing and gloves. |
| WGK Germany | 2 |
| HS Code | 29339900 |
Introduction
(S)-(-)-Indoline-2-carboxylic acid, chemically known as (S)-(-)-Indoline-2-carboxylic acid, is an organic compound.
Quality:
(S)-(-)-indolin-2-carboxylic acid is a colorless crystal with special structural and chiral characteristics. It has two stereoisomers, which are (S)-(-)-indolin-2-carboxylic acid and (R)-(+)-indoldoline-2-carboxylic acid.
Use:
(S)-(-)-indolin-2-carboxylic acid is widely used in organic synthesis. It is an important intermediate in the preparation of indoline compounds. It is also commonly used in the preparation of catalysts and stereoisomers for chiral synthesis.
Method:
(S)-(-)-indolin-2-carboxylic acid can usually be prepared by chiral synthesis. A common method is to use chiral derivatives for asymmetric reactions, such as asymmetric Yongji-Bodhi oxidation of pyridine using a chiral denitrification catalyst to obtain (S)-(-)-indolline-2-carboxylic acid.
Safety Information:
(S)-(-)-Indoline-2-carboxylic acid has low toxicity under conventional operating conditions. However, as an organic compound, it can have an irritating effect on the skin, eyes, and respiratory tract, and direct contact should be avoided and good ventilation should be maintained. Laboratory safety operating procedures should be strictly adhered to, and the compound should be stored and handled properly. In any case, it should be avoided by ingesting or inhaling. In case of skin contact or inhalation, wash immediately or call first aid.


![2-Amino-5-Cbz-4,5,6,7-tetrahydro-1,3-thiazolo[5,4-c]pyridine(CAS#1141669-69-3)](https://www.xinchem.com/uploads/AminoCbz.gif)