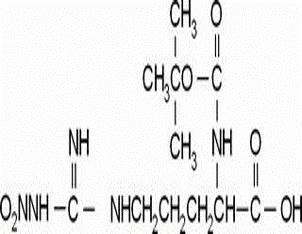
Romiplostim(CAS#267639-76-9)
Romiplostim(CAS#267639-76-9)
Pharmacological mechanism: It is an Fc-peptide fusion protein that increases platelet production by binding and activating TPO receptors, and its effect is similar to that of endogenous TPO, but the amino acid sequence is not homologous to endogenous thrombopoietin.
Pharmacokinetics: Patients with chronic immune thrombocytopenic purpura (ITP) receive weekly subcutaneous injections that peak at about 7 to 50 hours and have a half-life of 1 to 34 days, blood concentrations vary widely and disproportionately to dose, and elimination of romiplostim in serum is partially dependent on TPO receptors on platelets.
Indications: For the treatment of thrombocytopenia in patients with chronic idiopathic thrombocytopenic purpura (ITP), it should only be used if corticosteroids, immunoglobulin, or splenectomy are ineffective. In addition, it is also suitable for increasing the survival rate of adult and pediatric patients (including term neonates) who are acutely exposed to myelosuppressive dose radiation, and was approved for the first time in the world by the Japanese PMDA on September 25, 2023 for the treatment of aplastic anaemia.
Dosage: The initial dose is 1 μg/kg, subcutaneously 1 time per week, adjust the dose according to platelet count, the maximum dose should not exceed 10 μg/kg, 1 subcutaneous injection per week.
Adverse reactions: common headaches, joint pain, dizziness, insomnia, muscle pain, abdominal pain, etc. Serious adverse effects include bone marrow reticulum hard protein deposition, worsening of thrombocytopenia after discontinuation of romiplostim, and erythromelalgia, anaphylaxis, and angioedema may also occur.
Contraindications and caution: Thrombocytopenia due to myelodysplastic syndrome (MDS) or other non-ITP causes is contraindicated. ITP should be used with caution in patients with chronic liver disease, pregnant women, and patients with liver and kidney insufficiency.
History & Availability: In 1997, scientists identified the peptide and made it into romiplastim. Romliplastim was approved by the Australian Therapeutic Goods Administration (TGA) for ITP in July 2008 and by the U.S. Food and Drug Administration (FDA) in August. In February 2009, it was approved by the European Commission (EC) for the treatment of chronic idiopathic thrombocytopenic purpura. In December 2018, the US FDA approved it for pediatric patients ≥ 1 year of age, with an ITP duration of > 6 months and an inadequate response to corticosteroids, immunoglobulin, or splenectomy. In October 2019, it was approved by the U.S. FDA for early use in adults with immune thrombocytopenia. In January 2022, romiplostim was approved for marketing in China.