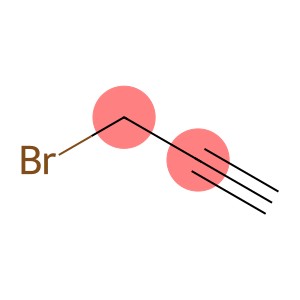
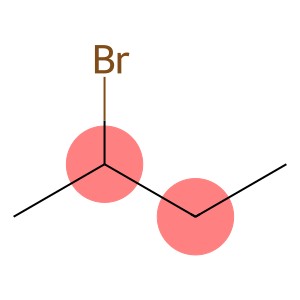
Propargyl bromide(CAS#106-96-7)
| Risk Codes | R60 – May impair fertility R61 – May cause harm to the unborn child R20/21 – Harmful by inhalation and in contact with skin. R25 – Toxic if swallowed R63 – Possible risk of harm to the unborn child R36/37/38 – Irritating to eyes, respiratory system and skin. R11 – Highly Flammable R67 – Vapors may cause drowsiness and dizziness R65 – Harmful: May cause lung damage if swallowed R48/20 - |
| Safety Description | S53 – Avoid exposure – obtain special instructions before use. S16 – Keep away from sources of ignition. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S37/39 – Wear suitable gloves and eye/face protection S28A - S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S62 – If swallowed, do not induce vomitting; seek medical advice immediately and show this container or label. S36/37 – Wear suitable protective clothing and gloves. |
| UN IDs | UN 2345 3/PG 2 |
| WGK Germany | 3 |
| RTECS | UK4375000 |
| FLUKA BRAND F CODES | 8 |
| TSCA | Yes |
| HS Code | 29033990 |
| Hazard Note | Highly Flammable/Toxic/Corrosive |
| Hazard Class | 3 |
| Packing Group | II |
Introduction
3-Bromopropyne, also known as 1-bromo-2-propyne, is an organic compound. The following is a brief introduction to its properties, uses, manufacturing methods and safety information:
Quality:
- It has a lower density, with a value of about 1.31 g/mL.
- 3-Bropropyne has a pungent odor.
- It can be soluble in some organic solvents such as ethanol and ether.
Use:
- 3-Broproyne is mainly used as a reagent in organic synthesis reactions, for example it can participate in metal-catalyzed cross-coupling reactions for the synthesis of organic compounds.
- It can also be used as a starting material for alkynes, e.g. for the synthesis of alkynes or other functionalized alkynes.
Method:
- 3-Bromopropyne can be obtained by the reaction of bromoacetylene and ethyl chloride under alkaline conditions.
- This is done by mixing bromoacetylene and ethyl chloride and adding a certain amount of alkali (such as sodium carbonate or sodium bicarbonate).
- At the end of the reaction, pure 3-bromopropynne is obtained by distillation and purification.
Safety Information:
- 3-Bropropyne is a toxic and irritating substance that requires proper personal protective equipment (PPE) to be worn when operating.
- It should avoid contact with oxidants, strong alkalis, and strong acids to avoid dangerous reactions.
- Comply with relevant safety operating procedures during use and storage.
- When handling 3-bromopropyne, ensure good ventilation and avoid inhaling its vapours or coming into contact with the skin and eyes.