Potassium trifluoroacetate(CAS# 2923-16-2)
| Risk Codes | R36/37/38 – Irritating to eyes, respiratory system and skin. R50 – Very Toxic to aquatic organisms R28 – Very Toxic if swallowed |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S22 – Do not breathe dust. S20 – When using, do not eat or drink. S37 – Wear suitable gloves. |
| UN IDs | 3288 |
| WGK Germany | 3 |
| FLUKA BRAND F CODES | 3-10 |
| TSCA | No |
| HS Code | 29159000 |
| Hazard Note | Irritant/Hygroscopic |
| Hazard Class | 6.1 |
| Packing Group | II |
Introduction
Potassium trifluoroacetate is an inorganic compound. It is a colorless crystalline or white powdery solid that is soluble in water and alcohol. The following is an introduction to the properties, uses, preparation methods and safety information of potassium trifluoroacetate:
Quality:
- Potassium trifluoroacetate is highly corrosive and reacts quickly with water and releases toxic hydrogen fluoride gas.
- It is a strong acidic substance that reacts with alkali to produce the corresponding salt.
- It can be oxidized by oxidizing agents to potassium oxide and carbon dioxide.
- Decomposes at high temperatures to produce toxic oxides and fluorides.
- Potassium trifluoroacetate has a corrosive effect on metals and can form fluoride with metals such as copper and silver.
Use:
- Potassium trifluoroacetate is widely used as a catalyst in organic synthesis reactions, especially in fluorination reactions.
- It can be used as an electrolyte additive in ferromanganese batteries and electrolytic capacitors.
- Potassium trifluoroacetate can also be used in metal surface treatment to improve the corrosion resistance of metal surfaces.
Method:
- Potassium trifluoroacetate can be formed by the reaction of trifluoroacetic acid with alkali metal hydroxides.
Safety Information:
- Potassium trifluoroacetate is irritating and should be avoided from contact with skin and eyes.
- Protective gloves, safety glasses and protective clothing should be worn during operation.
- Avoid inhaling its dust or vapour and should use it in a well-ventilated area.


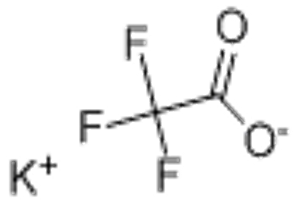
![2-(Chloromethyl)[1,3]oxazolo[4,5-b]pyridine(CAS#110704-34-2)](https://www.xinchem.com/uploads/oxazolopyridine.png)