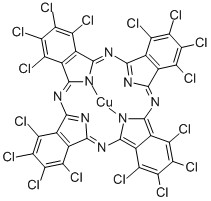
Pigment Green 7 CAS 1328-53-6
| Safety Description | 24/25 – Avoid contact with skin and eyes. |
| HS Code | 32041200 |
| Toxicity | LD50 oral in rat: > 10gm/kg |
Pigment Geen 7 CAS 1328-53-6 Information
quality
Phthalocyanine green G, also known as malachite green, is a common organic dye with the chemical formula C32Cl16CuN8. It has a vivid green color in solution and has the following properties:
1. Stability: Phthalocyanine Green G is a relatively stable compound that is not easy to decompose. It can be stored for long periods of time at normal temperatures and pressures, making it suitable for use as dyes and pigments.
2. Solubility: Phthalocyanine green G has good solubility in organic solvents, such as methanol, dimethyl sulfoxide and dichloromethane. But it has less solubility in water.
3. Light absorption: Phthalocyanine green G has strong light absorption properties, it has an absorption peak in the visible light band, and the maximum absorption peak is at about 622 nm. This absorbance makes phthalocyanine green G commonly used in analytical chemistry, biochemistry, and photosensitive materials.
4. Application: Due to its brilliant green color and stability, phthalocyanine green G is widely used in the preparation of dyes and pigments, such as fabrics, inks and plastics, etc. In addition, it is used for staining biological samples, fluorescent probes, and light-sensitive materials.
Uses and synthesis methods
Phthalocyanine Green G is an organic dye with a unique structure and properties. It is a green compound with the chemical name of copper phthalocyanine green. Phthalocyanine Green G is widely used in the fields of chemistry, materials and biological sciences.
The main uses of phthalocyanine green G are as follows:
1. Dyes: Phthalocyanine green G is a commonly used organic dye that can be used to color materials such as textiles, pigments, inks and plastics.
2. Scientific research: Phthalocyanine green G has important applications in chemical and biological science research, such as cell imaging, fluorescent probes and photosensitizers.
3. Optoelectronic devices: Phthalocyanine green G can be used to prepare organic optoelectronic devices, such as organic solar cells, field-effect transistors and organic light-emitting diodes.
There are many different synthesis routes for the synthesis of phthalocyanine green G, and one of the commonly used synthesis methods is as follows:
Phthalocyanine ketone is reacted with a solution containing copper ions to form a precursor of phthalocyanine green G. Then, the reaction conditions are adjusted by adding an appropriate amount of sodium hydroxide and amine compounds (such as methanolamine), which is further converted into phthalocyanine green G. Through filtrate, washing, drying and other steps, pure phthalocyanine green G product was obtained.
This is a common synthesis method of phthalocyanine green G, which can be adjusted and improved according to specific needs and conditions.