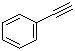
Phenylacetylene(CAS#536-74-3)
| Hazard Symbols | Xn – Harmful |
| Risk Codes | R10 – Flammable R36/37/38 – Irritating to eyes, respiratory system and skin. R40 – Limited evidence of a carcinogenic effect R65 – Harmful: May cause lung damage if swallowed |
| Safety Description | S16 – Keep away from sources of ignition. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) |
| UN IDs | UN 3295 |
Phenylacetylene(CAS#536-74-3) introduce
quality
Phenacetylene is an organic compound. Here are some of the properties of phenylacetylene:
1. Physical properties: Phenacetylene is a colorless liquid that is volatile at room temperature.
2. Chemical properties: Phenylacetylene can undergo many reactions related to carbon-carbon triple bonds. It can undergo an addition reaction with halogens, such as an addition reaction with chlorine to form phenylacetylene dichloride. Phenacetylene can also undergo a reduction reaction, reacting with hydrogen in the presence of a catalyst to form styrene. Phenylacetylene can also carry out the substitution reaction of ammonia reagents to generate the corresponding substitution products.
3. Stability: The carbon-carbon triple bond of phenylacetylene makes it have a high degree of unsaturation. It is relatively unstable and prone to spontaneous polymerization reactions. Phenacetylene is also highly flammable and should be avoided from contact with strong oxidizing agents and ignition sources.
These are some of the basic properties of phenylacetylene, which has important application value in organic synthesis, materials science and other fields.
Safety Information
Phenacetylene. Here is some safety information about phenylacetylene:
1. Toxicity: Phenylacetylene has a certain toxicity and can enter the human body by inhalation, contact with the skin, or ingestion. Long-term or high-concentration exposure may have adverse effects on the respiratory, nervous system, and liver.
2. Fire explosion: Phenylacetylene is a flammable substance that is capable of forming an explosive mixture with oxygen in the air. Exposure to open flames, high temperatures, or ignition sources can lead to fire or explosion. Contact with substances such as oxidants and strong acids should be avoided.
3. Avoid inhalation: Phenylacetylene has a pungent odor that may cause dizziness, drowsiness, and respiratory discomfort. Good ventilation should be maintained during operation and direct inhalation of phenylacetylene vapors or gases should be avoided.
4. Contact protection: When handling phenylacetylene, wear protective gloves, goggles and appropriate protective clothing to avoid contact with skin and eyes.
5. Storage and handling: Phenylacetylene should be stored in a cool, well-ventilated place, away from fire sources and open flames. The container should be inspected for intact condition before use. The handling process should follow safe operating procedures to avoid sparks and electrostatic charges.
Uses and synthesis methods
Phenacetylene is an organic compound. It is made up of a benzene ring linked to an acetylene group (EtC≡CH).
Phenacetylene has a wide range of applications in organic synthesis. Here are some of the main uses:
Pesticide synthesis: phenylacetylene is an important intermediate in the synthesis of some commonly used pesticides, such as dichlor.
Optical applications: Phenylacetylene can be used in photopolymerization reactions, such as the preparation of photochromic materials, photoresistive materials, and photoluminescent materials.
The synthesis methods of phenylacetylene in laboratories and industries are mainly as follows:
Acetylene reaction: through the arylation reaction and acetylenylation reaction of the benzene ring, the benzene ring and the acetylene group are connected to prepare phenylacetylene.
Enol rearrangement reaction: The enol on the benzene ring is reacted with acetylenol, and the rearrangement reaction occurs to produce phenylacetylene.
Alkylation reaction: the benzene ring is placed on