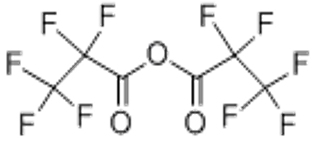Pentafluoropropionic anhydride(CAS# 356-42-3)
| Hazard Symbols | C – Corrosive |
| Risk Codes | R34 – Causes burns R14 – Reacts violently with water |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) |
| UN IDs | UN 3265 8/PG 2 |
| WGK Germany | 3 |
| FLUKA BRAND F CODES | 10-21 |
| TSCA | T |
| HS Code | 29159000 |
| Hazard Note | Corrosive |
| Hazard Class | 8 |
| Packing Group | II |
Introduction
Quality:
Pentafluoropropionic anhydride is a colorless to pale yellow liquid with a pungent odor. It is insoluble in water at room temperature, soluble in organic solvents such as ethanol, acetone, etc. It is a flammable liquid and is flammable.
Use:
Pentafluoropropionic anhydride is widely used in fluorination reactions in organic synthesis reactions and is often used as a substitute for hydrofluoric acid.
Method:
The preparation method of pentafluoropropionic anhydride is more complicated, and a common method is to react fluoroethanol with bromoacetic acid to form fluoroethyl acetate, and then deether it to obtain pentafluoropropionic anhydride.
Safety Information:
Pentafluoropropionic anhydride is irritating and may cause irritation of the eyes, respiratory tract and skin when inhaled, ingested, or in contact with the skin. Inhalation of its vapors should be avoided when used or operated. Necessary safety measures should be taken, such as wearing appropriate protective eyewear and gloves, and ensuring that they are used in a well-ventilated area. When carrying out fluorination reactions, the reaction conditions should be strictly controlled to avoid the production of harmful fluoride wastes.