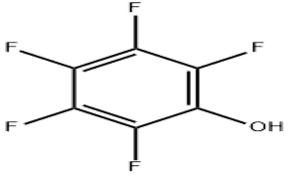
Pentafluorophenol (CAS# 771-61-9)
| Risk Codes | R36/37/38 – Irritating to eyes, respiratory system and skin. R34 – Causes burns R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. R63 – Possible risk of harm to the unborn child R43 – May cause sensitization by skin contact R23/24/25 – Toxic by inhalation, in contact with skin and if swallowed. R45 – May cause cancer R67 – Vapors may cause drowsiness and dizziness R40 – Limited evidence of a carcinogenic effect |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S36/37 – Wear suitable protective clothing and gloves. S24/25 – Avoid contact with skin and eyes. S23 – Do not breathe vapour. S53 – Avoid exposure – obtain special instructions before use. |
| UN IDs | 2811 |
| WGK Germany | 3 |
| RTECS | SM6680000 |
| FLUKA BRAND F CODES | 3 |
| TSCA | T |
| HS Code | 29081000 |
| Hazard Note | Toxic/Irritant |
| Hazard Class | 8 |
| Packing Group | III |
| Toxicity | LD50 scu-rat: 322 mg/kg IZSBAI 3,91,65 |
Introduction
Pentafluorophenol is an organic compound. It has the following properties:
1. Appearance: colorless crystalline solid.
4. Solubility: soluble in organic solvents such as ethanol, dimethylformamide, etc., slightly soluble in water.
5. Pentafluorophenol is a strong acidic substance, corrosive and irritating.
The main uses of pentafluorophenol are as follows:
1. Fungicide: Pentafluorophenol can be used for disinfection and sterilization, and has a strong inhibitory effect on bacteria, fungi and viruses. It is commonly used for hygienic disinfection in medical, laboratory and industrial applications.
3. Chemical reagents: pentafluorophenol can be used as reagents and reagent intermediates in organic synthesis.
Pentafluorophenol can be produced by the reaction of pentafluorobenzene with an alkaline oxidant such as sodium peroxide. The specific reaction equation is:
C6F5Cl + NaOH + H2O2 → C6F5OH + NaCl + H2O
The safety information of pentafluorophenol is as follows:
1. Skin and eye irritation: Pentafluorophenol has strong irritation, and contact with the skin or eyes will cause pain, redness and swelling and other uncomfortable symptoms.
2. Inhalation hazards: The vapor of pentafluorophenol has an irritating effect on the respiratory tract, and excessive inhalation may cause symptoms such as cough and difficulty breathing.
3. Ingestion hazards: Pentafluorophenol is considered toxic, and excessive ingestion may lead to toxic reactions.
When using pentafluorophenol, appropriate protective measures should be taken, such as wearing protective gloves, face shields, etc., and maintaining a well-ventilated working environment.