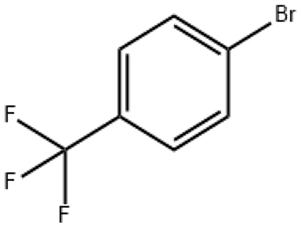
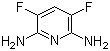
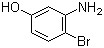
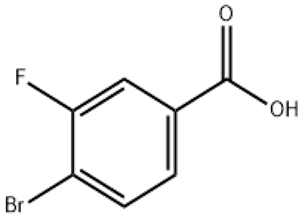
P-Bromobenzotrifluoride(CAS# 402-43-7)
| Risk Codes | R10 – Flammable R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S37/39 – Wear suitable gloves and eye/face protection S16 – Keep away from sources of ignition. |
| UN IDs | UN 1993 3/PG 3 |
| WGK Germany | 3 |
| TSCA | T |
| HS Code | 29036990 |
| Hazard Note | Flammable |
| Hazard Class | 3 |
| Packing Group | III |
Introduction
Bromotrifluorotoluene is an organic compound. It is a colorless and transparent liquid that has a very strong pungent odor at room temperature.
Bromotrifluorotoluene is mainly used as a donor of bromine atoms in organic synthesis reactions. It can react with aniline to generate substituted bromoaniline compounds, which have important applications in the pharmaceutical industry and pesticide synthesis. Bromotrifluorotoluene can also be used as a strong fluorinating agent in fluorination reactions.
A common method for the preparation of bromotrifluorotoluene is to hydrogenate bromine and trifluorotoluene in the presence of a catalyst. Another method is to pass bromine gas through trifluoromethyl compounds.
Inhalation of its vapours should be avoided when in use, and it should be ensured that the operation is carried out in a well-ventilated area. Bromotrifluorotoluene is also a flammable substance and should be kept away from fire and high temperatures. When encountering strong oxidizing agents, a violent reaction may occur, and separation from them should be maintained.