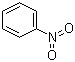
Nitrobenzene(CAS#98-95-3)
| Risk Codes | R23/24/25 – Toxic by inhalation, in contact with skin and if swallowed. R40 – Limited evidence of a carcinogenic effect R48/23/24 - R51/53 – Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. R62 – Possible risk of impaired fertility R39/23/24/25 - R11 – Highly Flammable R36/37/38 – Irritating to eyes, respiratory system and skin. R60 – May impair fertility R52/53 – Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment. R48/23/24/25 - R36 – Irritating to the eyes R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. |
| Safety Description | S28 – After contact with skin, wash immediately with plenty of soap-suds. S36/37 – Wear suitable protective clothing and gloves. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. S28A - S16 – Keep away from sources of ignition. S7 – Keep container tightly closed. S27 – Take off immediately all contaminated clothing. S53 – Avoid exposure – obtain special instructions before use. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| UN IDs | UN 1662 6.1/PG 2 |
| WGK Germany | 2 |
| RTECS | DA6475000 |
| TSCA | Yes |
| HS Code | 29042010 |
| Hazard Class | 6.1 |
| Packing Group | II |
| Toxicity | LD50 orally in rats: 600 mg/kg (PB91-108398) |
Introduction
Nitrobenzene) is an organic compound that can be a white crystalline solid or a yellow liquid with a special aroma. The following is an introduction to some of the properties, uses, preparation methods and safety information of nitrobenzene:
Quality:
Nitrobenzene is insoluble in water but soluble in organic solvents such as alcohols and ethers.
It can be obtained by nitrating benzene, which is produced by reacting benzene with concentrated nitric acid.
Nitrobenzene is a stable compound, but it is also explosive and has a high flammability.
Use:
Nitrobenzene is an important chemical raw material and is widely used in organic synthesis.
Nitrobenzene can also be used as an additive in solvents, paints and coatings.
Method:
The preparation method of nitrobenzene is mainly obtained by the nitrification reaction of benzene. In the laboratory, benzene can be mixed with concentrated nitric acid and concentrated sulfuric acid, stirred at low temperatures, and then rinsed with cold water to obtain nitrobenzene.
Safety Information:
Nitrobenzene is a toxic compound, and exposure to or inhalation of its vapor can cause damage to the body.
It is a flammable and explosive compound and should avoid contact with ignition sources.
Personal protective equipment such as protective gloves and goggles should be worn when handling nitrobenzene, and a well-ventilated operating environment should be maintained.
In the event of a leak or accident, appropriate measures should be taken promptly to clean up and dispose of it. Comply with relevant laws and regulations to properly dispose of the waste generated.