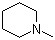
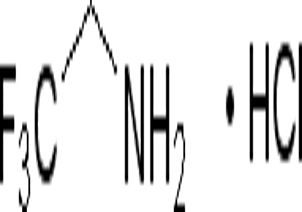N-Methylpiperidine(CAS#626-67-5)
| Risk Codes | R11 – Highly Flammable R34 – Causes burns R20/22 – Harmful by inhalation and if swallowed. R39/23/24/25 - R23/24/25 – Toxic by inhalation, in contact with skin and if swallowed. R67 – Vapors may cause drowsiness and dizziness R22 – Harmful if swallowed R10 – Flammable R37 – Irritating to the respiratory system |
| Safety Description | S7 – Keep container tightly closed. S16 – Keep away from sources of ignition. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S9 – Keep container in a well-ventilated place. S33 – Take precautionary measures against static discharges. |
| UN IDs | UN 3286 3/PG 2 |
| WGK Germany | 3 |
| RTECS | TN1225000 |
| FLUKA BRAND F CODES | 3-10 |
| TSCA | Yes |
| HS Code | 29333999 |
| Hazard Class | 3 |
| Packing Group | II |
| Toxicity | LD50 intraperitoneal in mouse: 400mg/kg |
Introduction
N-methylpiperidine is an organic compound also known as N-methylpiperidine.N-methylpiperidine is a colorless liquid with a pungent odor. At room temperature, it is relatively stable, but reacts with oxygen, acids, and oxidants. N-methylpiperidine is also an alkaline substance that can react with acids to form salts.N-methylpiperidine has a wide range of applications in organic synthesis. It is often used as a catalyst and solvent to facilitate a variety of chemical reactions.There are two main ways to prepare N-methylpiperidine. One is synthesized by methylation of piperidine, which can be done with reagents such as ammonium methyl chloride or ammonium methyl iodide. The other is the formation of N-methylpiperidine by the reaction of ammonium cyanoformate and benzylamine.It has a pungent odor and vapor and should provide good ventilation conditions when operating. Contact with skin and eyes should be avoided, and rinsed immediately with plenty of water if contact occurs. When storing, it should be kept tightly sealed, away from fire sources and oxidants.