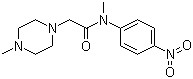
“N-Methyl-2-(4-methylpiperazin-1-yl)-N- (4-nitrophenyl)acetamide(CAS#1139453-98-7)”
N-Methyl-2-(4-methylpiperazin-1-yl)-N- (4-nitrophenyl)acetamide(CAS#1139453-98-7)
Physicochemical properties
Appearance: Usually solid, but the exact appearance may vary depending on factors such as synthesis method and purity.
Solubility: Its solubility can be affected by both polar and non-polar groups in the molecule. Generally speaking, it has good solubility in organic solvents such as dichloromethane, chloroform, N,N-dimethylformamide (DMF), etc., while the solubility in water may be relatively poor, but the specific solubility data need to be further determined by experiments.
Stability: Under normal temperature and pressure, it should be relatively stable under appropriate storage conditions. However, due to the presence of functional groups such as nitro in the molecule, it may have a certain sensitivity to heat, light, acid, alkali and other conditions. For example, nitro groups may undergo a reduction reaction under certain conditions, etc.
Synthesis method
In general, N-(4-nitrophenyl)chloroacetamide can be formed by reacting with chloroacetyl chloride with 4-nitroaniline as the starting material, followed by nucleophilic substitution reaction with N-methylpiperazine, and finally introducing methyl groups through methylation reaction to obtain the target product. Of course, the actual synthetic route may be optimized and adjusted according to the specific experimental conditions and requirements, and may also involve strategies such as the use of protective groups to ensure the selectivity and yield of the reaction.
Fields of application
Pharmaceutical: Such compounds containing piperazine and nitrophenyl structures often have potential biological activity and may have antibacterial, antiviral, antitumor, or other pharmacological effects. For example, piperazine groups can interact with certain target proteins or enzymes in organisms, while nitrophenyl groups may be involved in hydrogen bonding or other specific binding to targets to exert pharmacological activity, but the specific biological activity needs to be determined through a large number of experimental studies.
Organic synthesis intermediates: Due to the presence of a variety of functional groups in their structures, they can be used as important organic synthesis intermediates for the further synthesis of organic compounds with more complex structures and special functions. For example, it can be further functionalized and other active groups can be introduced to construct drug molecules or material molecules with specific properties.