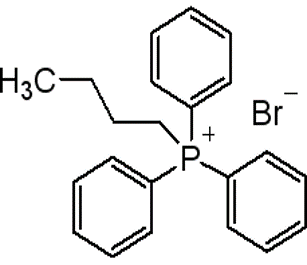
(n-Butyl)triphenylphosphonium bromide(CAS# 1779-51-7)
Risk and Safety
| Hazard Symbols | Xn – Harmful |
| Risk Codes | R21/22 – Harmful in contact with skin and if swallowed. R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. |
| UN IDs | 3464 |
(n-Butyl)triphenylphosphonium bromide(CAS# 1779-51-7)Uses and synthesis methods
Butyltriphenylphosphine bromide is an organophosphorus compound. It has important applications in organic synthesis, and here are some of its common uses and synthesis methods:
Use:
1. Catalyst: Butyltriphenylphosphine bromide is commonly used as a catalyst for certain chemical reactions. For example, in the Friedel-Gram reaction, it can catalyze the coupling reaction between alkynes and borides to synthesize topological isomers of alkynes.
2. Organometallic chemistry: Butyltriphenylphosphine bromide can also be used as a ligand in organometallic chemistry. It can form complexes with metal ions and participate in some important organic synthesis reactions, such as Suzuki reaction.
Synthesis Method:
There are several methods for the synthesis of butyltriphenylphosphine bromide, and the following is one of the common methods:
1. Reaction raw materials: bromobenzene, triphenylphosphine, butane bromide;
2. Steps:
(1) In an inert atmosphere, bromobenzene and triphenylphosphine are added to the reaction flask;
(2) The reaction bottle is sealed and stirred under temperature control, and the general reaction temperature is 60-80 degrees Celsius;
(3) Slowly add butane bromide as needed and continue the reaction stirring;
(4) After the reaction is completed, cool to room temperature;
(5) Extraction and washing with solvents, and drying, crystallization and other treatment steps;
(6) Finally, butyltriphenylphosphine bromide product is obtained.