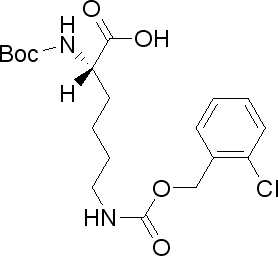
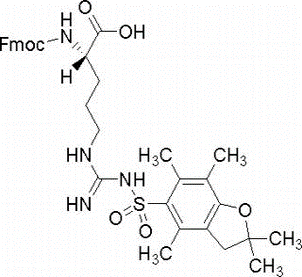
N-Boc-N’-(2-chlorobenzyloxycarbonyl)-L-lysine(CAS# 54613-99-9)
| Hazard Symbols | Xi – Irritant |
| Risk Codes | 36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S24/25 – Avoid contact with skin and eyes. |
| WGK Germany | 3 |
| HS Code | 29242990 |
Introduction
N-tert-butoxycarbonyl-N’-(2-chlorobenzyloxycarbonyl)-L-lysine is an organic compound, commonly referred to as CBZ-L-lysine. The following is the nature, use, preparation method and safety information of the compound:
Quality:
CBZ-L-lysine is a colorless crystalline solid with a peculiar odor. It has good solubility and is soluble in common organic solvents such as methanol, chloroform, and dimethyl sulfoxide.
Use:
CBZ-L-lysine is often used as one of the amino protective groups in organic synthesis to protect the amino functional groups that are sensitive to the environment. In the synthesis of peptide compounds, CBZ-L-lysine can be used to protect the amino group of lysine in order to protect or control its reactivity in specific reactions.
Method:
The preparation of CBZ-L-lysine is usually carried out by the following steps: L-lysine is reacted with carbon dioxide to obtain the corresponding carbonate; Then, the carbonate is reacted with tert-butoxycarbonyl magnesium chloride to obtain acetyl-protected lysine; It is then reacted with 2-chlorobenzyl iodine chloride and alkali to obtain CBZ-L-lysine.
Safety Information:
The use of CBZ-L-lysine should be accompanied by the following safety precautions: it may be irritating to the eyes, skin and respiratory tract, and direct contact should be avoided during operation. Appropriate protective equipment such as chemical protective glasses and gloves should be worn during use. It should be operated in a well-ventilated area to avoid inhaling vapors from the compound. If an accident occurs, the affected area should be rinsed immediately with plenty of water and medical assistance should be sought.