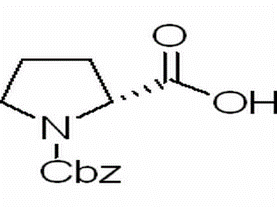
N-Benzyloxycarbonyl-D-proline(CAS# 6404-31-5)
| Hazard Symbols | Xi – Irritant |
| Safety Description | 24/25 – Avoid contact with skin and eyes. |
| WGK Germany | 3 |
| HS Code | 29339900 |
Introduction
N-Benzyloxycarbonyl-D-proline is an organic compound whose chemical formula is C14H17NO4. The following is a description of its nature, use, preparation and safety information:
Nature:
N-Benzyloxycarbonyl-D-proline is a white solid soluble in organic solvents. It has a relatively high melting point and boiling point, and is a non-volatile compound. It is partially soluble in water. The compound is a chiral molecule with D-configuration.
Use:
N-Benzyloxycarbonyl-D-proline is often used as a reagent to protect amino acids in organic synthesis. By reacting with an amino acid, a stable N-benzyloxycarbonyl protecting group can be formed to prevent other reactions from occurring. Subsequently, the target compound can be obtained by a method of selectively deprotecting the group.
Preparation Method:
Generally, the preparation method of N-Benzyloxycarbonyl-D-proline involves the following steps:
1. D-proline reacts with benzyl alcohol to generate N-Benzyloxycarbonyl-D-proline benzyl ester.
2. The proline benzyl ester is esterified to N-Benzyloxycarbonyl-D-proline by acid or base catalysis.
Safety Information:
N-Benzyloxycarbonyl-D-proline safety data is limited, but necessary precautions should be taken in accordance with general laboratory safety practices. This includes wearing glasses, laboratory coats and gloves, and avoiding inhalation and skin contact during use. In addition, it should be operated in a well-ventilated area and comply with local laws and regulations. In case of accidental contact, rinse immediately with plenty of water and seek medical assistance.


![4,5,6,7-Tetrahydrobenzo[d]isoxazol-3-ylaMine)(CAS#1004-64-4)](https://www.xinchem.com/uploads/disoxazol.gif)