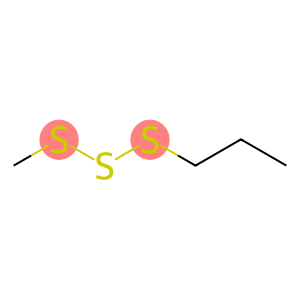
Methyl propyl trisulphide(CAS#17619-36-2)
| Hazard Symbols | Xi – Irritant |
| Risk Codes | R36/37/38 – Irritating to eyes, respiratory system and skin. R52/53 – Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. |
| WGK Germany | 3 |
Introduction
Methylpropyl trisulfide is an organic sulfide. The following is an introduction to the properties, uses, preparation methods and safety information of methylpropyl trisulfide:
Quality:
- Appearance: Methylpropyl trisulfide is a colorless to pale yellow liquid.
- Solubility: Soluble in organic solvents such as ethanol and ether.
- Aroma: with a pronounced sulfide odor.
Use:
- Methylpropyl trisulfide is mainly used as a rubber accelerator to improve the tensile strength and wear resistance of rubber.
- Methylpropyl trisulfide is also used in the preparation of certain vulcanized rubbers and adhesives.
Method:
- The preparation of methylpropyl trisulfide can be achieved by the use of sulfur in the presence of cuprous chloride and tributyltin in reaction with pentylene glycol.
Safety Information:
- Methylpropyl trisulfide has a pungent odor and may cause irritation to the eyes and respiratory system.
- Wear appropriate personal protective equipment, including protective eyewear and masks, when in use.
- Avoid contact with the skin, and if it does, rinse immediately with plenty of water. If you feel unwell, you should seek medical attention immediately.
- Methylpropyl trisulfide should be stored in a dry and ventilated place away from contact with oxygen, acids, or oxidizing agents.