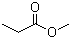Methyl propionate(CAS#554-12-1)
| Risk Codes | R11 – Highly Flammable R20 – Harmful by inhalation R2017/11/20 - |
| Safety Description | S16 – Keep away from sources of ignition. S24 – Avoid contact with skin. S29 – Do not empty into drains. S33 – Take precautionary measures against static discharges. |
| UN IDs | UN 1248 3/PG 2 |
| WGK Germany | 1 |
| RTECS | UF5970000 |
| TSCA | Yes |
| HS Code | 2915 50 00 |
| Hazard Class | 3 |
| Packing Group | II |
| Toxicity | LD50 orally in Rabbit: 5000 mg/kg |
Introduction
Methyl propionate, also known as methoxyacetate. The following is an introduction to the properties, uses, preparation methods and safety information of methyl propionate:
Quality:
- Appearance: Methyl propionate is a colorless transparent liquid with a special fragrance.
- Solubility: Methyl propionate is more soluble in anhydrous alcohols and ether solvents, but less soluble in water.
Use:
- Industrial use: Methyl propionate is an important organic solvent that is widely used in coatings, inks, adhesives, detergents and other industries.
Method:
The preparation of methyl propionate is often esterified:
CH3OH + CH3COOH → CH3COOCH2CH3 + H2O
Among them, methanol and acetic acid react under the action of a catalyst to form methyl propionate.
Safety Information:
- Methyl propionate is a flammable liquid and should be kept away from open flames and high temperatures.
- Exposure to methyl propionate may cause eye and skin irritation, so precautions should be taken.
- Avoid inhaling the vapor of methyl propionate and should operate in a well-ventilated area.
- In case of accidental ingestion or inhalation, seek medical attention immediately.