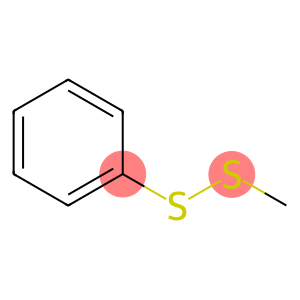
Methyl phenyl disulfide(CAS#14173-25-2)
| Hazard Symbols | Xi – Irritant |
| Risk Codes | R36/37/38 – Irritating to eyes, respiratory system and skin. R10 – Flammable |
| Safety Description | S37/39 – Wear suitable gloves and eye/face protection S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S16 – Keep away from sources of ignition. |
| HS Code | 29309099 |
Introduction
Methylphenyl disulfide (also known as methyldiphenyl disulfide) is an organic compound. The following is an introduction to the properties, uses, preparation methods and safety information of methylphenyl disulfide:
Quality:
- Appearance: Colorless to light yellow liquid
- Odor: There is a peculiar sulfide odor
- Flash Point: Approximately 95°C
- Solubility: Soluble in organic solvents such as ethanol and dimethylformamide
Use:
- Methylphenyl disulfide is commonly used as a vulcanization accelerator and crosslinker.
- It is commonly used in the rubber industry for the vulcanization reaction of rubber, which can improve the wear resistance, heat resistance and physical and mechanical properties of rubber.
- Methylphenyl disulfide can also be used in the preparation of chemicals such as dyes and pesticides.
Method:
Methylphenyl disulfide can be prepared by the reaction of diphenyl ether and mercaptan. The specific process is as follows:
1. In an inert atmosphere, diphenyl ether and mercaptan are slowly added to the reactor at an appropriate molar ratio.
2. Add an acidic catalyst (e.g., trifluoroacetic acid) to facilitate the reaction. The reaction temperature is generally controlled at room temperature or slightly higher temperature.
3. After the end of the reaction, the desired methylphenyl disulfide product is separated by distillation and purification.
Safety Information:
- Methylphenyl disulfide is an organic sulfide that may cause some irritation and toxicity to the human body.
- Wear appropriate protective gloves, goggles, and gas masks when operating to avoid contact with skin and inhalation of gases.
- Avoid contact with strong oxidizing agents and acids to avoid dangerous reactions.
- Keep away from ignition sources to avoid static sparks.
- Follow proper storage and handling practices to avoid accidents.