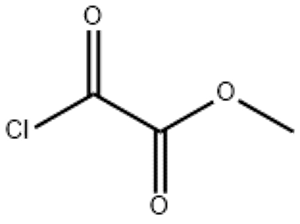
Methyl chloroglyoxylate(CAS# 5781-53-3)
| Risk Codes | R34 – Causes burns R37 – Irritating to the respiratory system R10 – Flammable R36 – Irritating to the eyes R14 – Reacts violently with water |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S16 – Keep away from sources of ignition. |
| UN IDs | UN 2920 8/PG 2 |
| WGK Germany | 3 |
| FLUKA BRAND F CODES | 9-21 |
| TSCA | Yes |
| HS Code | 29171900 |
| Hazard Class | 8 |
| Packing Group | II |
Introduction
Methyloxaloyl chloride is an organic compound. The following is an introduction to its nature, use, preparation method and safety information:
Quality:
Methyloxaloyl chloride is a colorless liquid with a pungent odor. It is a strong acidic substance that reacts with water to form formic acid and oxalic acid. Methyl oxaloyl chloride has high vapor pressure and volatility, and at the same time has strong corrosiveness.
Use:
Methyl oxaloyl chloride is an important intermediate in organic synthesis. Oxalyl methyl chloride can be used for a variety of organic synthesis reactions, such as acylation reaction, esterification reaction and carboxylic acid derivative synthesis.
Method:
The preparation of methyl oxaloyl chloride often uses benzoic acid as raw material, and oxaloyl chloroformimide is generated under the action of thionyl chloride, and then hydrolyzed to obtain methyl oxaloyl chloride.
Safety Information:
Methyloxaloyl chloride is highly irritating and corrosive, and can cause chemical burns in contact with the skin and eyes. Direct contact should be avoided during use and storage. Appropriate protective gloves, protective eyewear and respiratory protective equipment should be worn when in use. Operate in a well-ventilated area and avoid inhaling its vapors. When storing, it should be stored separately from oxidants, acids and alkalis to prevent fires and accidents.