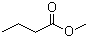
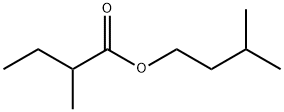
Methyl butyrate(CAS#623-42-7)
| Risk Codes | R20 – Harmful by inhalation R36/37/38 – Irritating to eyes, respiratory system and skin. R11 – Highly Flammable |
| Safety Description | S16 – Keep away from sources of ignition. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S33 – Take precautionary measures against static discharges. S29 – Do not empty into drains. S9 – Keep container in a well-ventilated place. |
| UN IDs | UN 1237 3/PG 2 |
| WGK Germany | 2 |
| RTECS | ET5500000 |
| FLUKA BRAND F CODES | 13 |
| TSCA | Yes |
| HS Code | 29156000 |
| Hazard Class | 3 |
| Packing Group | II |
Introduction
Methyl butyrate. The following is an introduction to some of the properties, uses, preparation methods and safety information of methyl butyrate:
Quality:
- Methyl butyrate is a flammable liquid that is less water-soluble.
- It has good solubility, soluble in alcohols, ethers and some organic solvents.
Use:
- Methyl butyrate is commonly used as a solvent, plasticizer and diluent in coatings.
- It can also be used as an intermediate in organic synthesis for the preparation of other compounds.
Method:
- Methyl butyrate can be prepared by reacting butyric acid with methanol under acidic conditions. The reaction equation is as follows:
CH3COOH + CH3OH → CH3COOCH2CH2CH3 + H2O
- The reaction is often carried out by heating with a catalyst (e.g., sulfuric acid or ammonium sulfate).
Safety Information:
- Methyl butyrate is a flammable liquid that can burn when exposed to open flames, high temperatures, or organic oxidants.
- Contact with skin and eyes may cause irritation and burns, precautions should be taken.
- Methyl butyrate has a certain toxicity, so it should be avoided for inhalation and accidental ingestion, and used under well-ventilated conditions.
- Care should be taken to prevent contact with oxidants, acids and alkalis when using or storing.