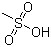
Methanesulfonic acid(CAS#75-75-2)
| Hazard Symbols | C – Corrosive |
| Risk Codes | R34 – Causes burns R22 – Harmful if swallowed R21/22 – Harmful in contact with skin and if swallowed. R35 – Causes severe burns |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S1/2 – Keep locked up and out of the reach of children. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. |
| UN IDs | UN 3265 8/PG 2 |
| WGK Germany | 1 |
| RTECS | PB1140000 |
| TSCA | Yes |
| HS Code | 29041000 |
| Hazard Class | 8 |
| Packing Group | III |
| Toxicity | LD50 orally in Rabbit: 649 mg/kg LD50 dermal Rabbit 200 – 2000 mg/kg |
Introduction
Methanesulfonic acid is an organic compound. The following is an introduction to the properties, uses, preparation methods and safety information of methanesulfonic acid:Quality:- Appearance: Methanesulfonic acid is a colorless to yellowish liquid.- Solubility: Soluble in water and most organic solvents.Use:- As a catalyst: Methanesulfonic acid can be used as a catalyst in organic synthesis, for example as a catalyst in carbohydrate chemical reactions.- Electrochemistry: Methanesulfonic acid can be used in electrochemical applications, such as as as a component in electrolytes to improve the performance of electrodes.Method:- Methanesulfonic acid can be prepared by reacting the chlorination byproduct produced by the methylsulfonyl chloride reaction with water.- Or it can be prepared by the reaction of methanesulfonic acid with hydrogen peroxide.Safety Information:- Methanesulfonic acid is a corrosive chemical that requires safe handling such as wearing chemical gloves, safety glasses, and protective clothing.- Keep away from fire and high temperatures when using or storing to avoid contact with oxidants.- Ensure a well-ventilated laboratory environment and avoid inhaling its vapors.These are basic introductions to methanesulfonic acid, please follow strict safety operating procedures when using or handling methanesulfonic acid.