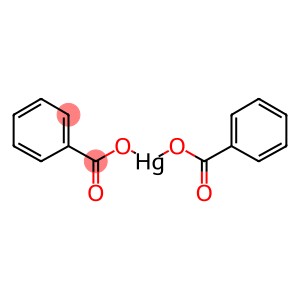
MERCURIC BENZOATE(CAS#583-15-3)
| Risk Codes | R26/27/28 – Very toxic by inhalation, in contact with skin and if swallowed. R33 – Danger of cumulative effects R50/53 – Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. |
| Safety Description | S13 – Keep away from food, drink and animal foodstuffs. S28 – After contact with skin, wash immediately with plenty of soap-suds. S36 – Wear suitable protective clothing. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S60 – This material and its container must be disposed of as hazardous waste. S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. |
| UN IDs | UN 1631 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | OV7060000 |
| Hazard Class | 6.1(a) |
| Packing Group | II |
Introduction
Mercury benzoate is an organic mercury compound with the chemical formula C14H10HgO4. It is a colorless crystalline solid that is stable at room temperature.
One of the main uses of mercury benzoate is as a catalyst for organic synthesis. It can be used to synthesize organic compounds such as alcohols, ketones, acids, etc. In addition, mercury benzoate can also be used in electroplating, fluorescents, fungicides, etc.
The preparation method of mercury benzoate is generally obtained by the reaction of benzoic acid and mercury hypochlorite (HgOCl). The following equations can be referred to in the specific preparation process:
C6H5CH2COOH + HgOCl → C6H5HgO2 + HCl + H2O
Pay attention to safety measures when using mercury benzoate. It is a highly toxic substance that can cause serious harm to human health if inhaled or in contact with the skin. Personal protective equipment such as gloves, goggles, and face shields should be worn when used and operated in a well-ventilated laboratory environment. When storing and transporting, contact with acids, oxides and other substances should be avoided to avoid dangerous reactions. Waste disposal should be carried out in accordance with relevant regulations. Under no circumstances should mercury benzoate come into direct contact with humans or the environment.