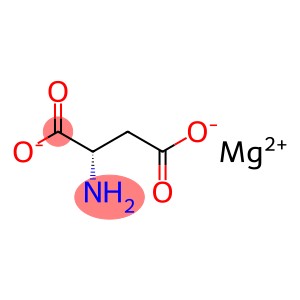
Magnesium-L-Aspartate CAS 2068-80-6
| Hazard Symbols | Xn – Harmful |
| Risk Codes | R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S22 – Do not breathe dust. S24/25 – Avoid contact with skin and eyes. S36 – Wear suitable protective clothing. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| WGK Germany | 2 |
Magnesium-L-Aspartate CAS 2068-80-6 Introduction
Brief introduction
Potassium aspartate is a salt compound. The following is an introduction to the properties, uses, preparation methods and safety information of potassium magnesium aspartate:
Quality:
Potassium magnesium aspartate was an orthorhombic crystal, and its unit cell parameters were a=0.7206 nm, b=1.1796 nm, and c=0.6679 nm.
Soluble in water and neutral in aqueous solution.
It has good chemical stability, high temperature resistance and light resistance.
Potassium aspartate is an important mineral in living organisms, which can be involved in biological processes such as enzyme catalysis and cell signaling.
Use:
Potassium magnesium aspartate has the functions of stabilizing mood, promoting sleep, and relieving stress, and is widely used in health care products to improve mood and enhance stress resistance.
Method:
The preparation method of potassium aspartate and magnesium is usually obtained by the reaction of aspartic acid and an appropriate amount of magnesium sulfate and potassium sulfate. The specific preparation method can be adjusted as needed.
Safety Information:
Potassium magnesium aspartate is generally considered relatively safe, but general laboratory practices and chemical safety routines should still be followed during use.
Avoid contact with strong acids or bases to avoid unwanted reactions.
Avoid prolonged contact with skin and wear gloves when using.