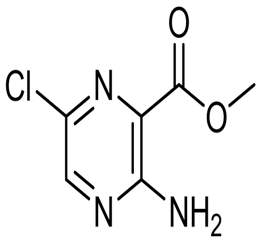
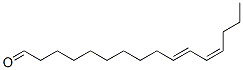
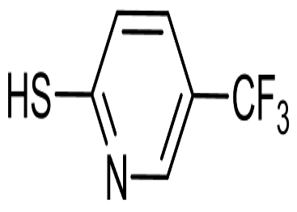
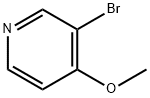
Lomefloxacin hydrochloride CAS 98079-52-8
Risk and Safety
| Hazard Symbols | Xn – Harmful |
| Risk Codes | 22 – Harmful if swallowed |
| WGK Germany | 3 |
| RTECS | VB1997500 |
| HS Code | 29339900 |
Content determination
Authoritative Data Verified Data
measured by high performance liquid chromatography (General 0512).
chromatographic conditions and system suitability test
silica gel bonded with octylsilane as filler; Sodium pentanesulfonate solution (sodium pentanesulfonate 1.5g, ammonium dihydrogen phosphate 3.0g, water 950ml to dissolve, adjust pH to with phosphoric acid, dilute to 1000ml with water)-Methanol (65:35) as mobile phase, flow rate of 1.2ml per minute, detection wavelength of 287mn. Take the system applicable solution 20u1 under the item of related substances and inject it into the human Liquid Chromatograph. The retention time of lomefloxacin is about 9 minutes, and the separation degree between the impurity peak at the relative retention time of about 0.8 and the lomefloxacin peak should be greater than 2.0, the resolution between lomefloxacin peak and impurity peak at relative retention time 1.1 should meet the requirements.
assay
take the right amount of this product, precision weighing, plus mobile phase dissolution and quantitative dilution made in each lml containing about 0. The lmg solution was used as the test solution, and 20ul was injected into the liquid chromatograph with precision, and the chromatogram was recorded. Another lomefloxacin reference substance was taken and determined by the same method. The content of lomefloxacin (C17H19F2N303) in the sample was calculated by peak area according to external standard method.