Lithium borohydride(CAS#16949-15-8)
| Risk Codes | R14/15 - R23/24/25 – Toxic by inhalation, in contact with skin and if swallowed. R34 – Causes burns R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. R11 – Highly Flammable R40 – Limited evidence of a carcinogenic effect R36/37/38 – Irritating to eyes, respiratory system and skin. R19 – May form explosive peroxides R67 – Vapors may cause drowsiness and dizziness R66 – Repeated exposure may cause skin dryness or cracking R22 – Harmful if swallowed R12 – Extremely Flammable |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S43 – In case of fire use … (there follows the type of fire-fighting equipment to be used.) S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36/37 – Wear suitable protective clothing and gloves. S16 – Keep away from sources of ignition. |
| UN IDs | UN 3399 4.3/PG 1 |
| WGK Germany | 2 |
| RTECS | ED2725000 |
| FLUKA BRAND F CODES | 10-21 |
| TSCA | Yes |
| HS Code | 2850 00 20 |
| Hazard Class | 4.3 |
| Packing Group | I |
Introduction
Lithium borohydride is an inorganic compound with the chemical formula BH4Li. It is a solid substance, usually in the form of a white crystalline powder. Lithium borohydride has the following properties:
1. High hydrogen storage capacity: Lithium borohydride is an excellent hydrogen storage material, which can store hydrogen at a high mass ratio.
2. Solubility: Lithium borohydride has high solubility and can be dissolved in many organic solvents, such as ether, ethanol and THF.
3. High flammability: Lithium borohydride can be burned in the air and release a large amount of energy.
The main uses of lithium borohydride are:
1. Hydrogen storage: Due to its high hydrogen storage capacity, lithium borohydride is widely used in the field of hydrogen energy to store and release hydrogen.
2. Organic synthesis: Lithium borohydride can be used as a reducing agent for hydrogenation reactions in organic chemical synthesis reactions.
3. Battery technology: Lithium borohydride can also be used as an electrolyte additive for lithium-ion batteries.
The preparation method of lithium borohydride is generally prepared by the reaction of lithium metal and boron trichloride. The specific preparation method is as follows:
1. Using anhydrous ether as a solvent, lithium metal is added to the ether in an inert atmosphere.
2. Add the ether solution of boron trichloride to the lithium metal.
3. Stirring and constant temperature reaction are carried out, and lithium borohydride is filtered after the reaction is completed.
1. Lithium borohydride is easy to burn when in contact with air, so avoid contact with open flames and high-temperature substances.
2. Lithium borohydride is irritating to the skin and eyes, and appropriate personal protective equipment such as gloves and goggles should be worn when operating.
3. Lithium borohydride should be stored in a dry place, away from water and humid environment, to prevent it from absorbing moisture and decomposing.
Please ensure that you have understood and mastered the correct operation methods and safety knowledge before using lithium borohydride. If you are unsafe or in doubt, you should seek professional guidance.


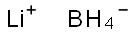
![4-[(4,6-dichloropyrimidin-2-yl)amino]benzonitrile(CAS#329187-59-9)](https://www.xinchem.com/uploads/dichloropyrimidin.png)