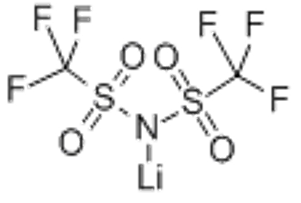Lithium bis(trifluoromethanesulphonyl)imide(CAS# 90076-65-6)
| Risk Codes | R24/25 - R34 – Causes burns R52/53 – Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment. R48/22 – Harmful danger of serious damage to health by prolonged exposure if swallowed. |
| Safety Description | S22 – Do not breathe dust. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. |
| UN IDs | UN 2923 8/PG 2 |
| WGK Germany | 2 |
| TSCA | Yes |
| HS Code | 29309090 |
| Hazard Note | Harmful/Corrosive/Moisture Sensitive |
| Hazard Class | 8 |
| Packing Group | II |
Introduction
Lithium bis-trifluoromethane sulfonimide. The following is an introduction to its nature, use, manufacturing methods and safety information:
Quality:
Lithium bis-trifluoromethane sulfonimide is a colorless crystal or white crystalline powder, which has high thermal and chemical stability. It is soluble in non-polar solvents such as ether and chloroform at room temperature, but it is difficult to dissolve in water.
Use:
Lithium bis-trifluoromethane sulfonimide is widely used in the field of organic synthesis. It can be used as a catalyst in strongly acidic systems and organic synthesis, such as fluoride ion sources and alkali catalysts in strongly alkaline systems. It can also be used as an electrolyte additive in lithium-ion batteries.
Method:
The preparation of lithium bis-trifluoromethane sulfonimide is generally obtained by reacting trifluoromethane sulfonimide with lithium hydroxide. Trifluoromethane sulfonimide is dissolved in a polar solvent, and then lithium hydroxide is added to generate lithium bistrifluoromethane sulfonimide during the reaction, and the product is subsequently obtained by concentration and crystallization.
Safety Information:
Lithium bis-trifluoromethane sulfonimide is generally safe under normal conditions of use, but there are still a few things to keep in mind:
- Lithium bistrifluoromethane sulfonimide can cause eye and skin irritation, and direct contact should be avoided during handling.
- Proper ventilation measures should be taken when handling, storing, or disposing of lithium bistrifluoromethane sulfonimide to ensure safety.
- When heated or exposed to high temperatures, lithium bistrifluoromethane sulfonimide is a risk of explosion and should be avoided from contact with open flames or high temperatures.
- When using lithium bis-trifluoromethane sulfonimide, follow the relevant safety operating procedures and wear appropriate personal protective equipment such as gloves, goggles, and protective clothing.