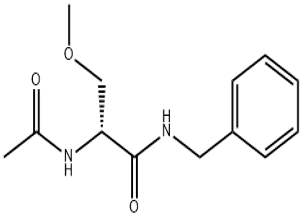
Lacosamide(CAS# 175481-36-4)
Risk and Safety
| Risk Codes | R11 – Highly Flammable R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. R36 – Irritating to the eyes |
| Safety Description | S16 – Keep away from sources of ignition. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37 – Wear suitable protective clothing and gloves. |
| UN IDs | UN 1648 3 / PGII |
| WGK Germany | 2 |
| HS Code | 2924296000 |
Lacosamide(CAS# 175481-36-4) introduction
Lactamide is a class of organic compounds containing lactam rings. The following is an introduction to the properties, uses, preparation methods and safety information of laclamide:
Quality:
The properties of laclamide depend on its molecular structure and the size of the ring. In general, lacamide is a white crystalline solid with strong stability. It has good solubility, soluble in organic solvents such as ethers and ketones, and insoluble in water.
Use:
Laccamide has a wide range of applications in the chemical industry. The most important of these is its use as a precursor to polymer materials. For example, polyamide fibers (nylon) are made by polymerizing laclamide. Laxamide can also be used as an intermediate in solvents, catalysts, and as a raw material for the manufacture of synthetic fibers, synthetic rubber, pharmaceuticals and dyes.
Method:
In general, the synthesis of laxamide is mainly achieved by acid-catalyzed cyclization. Specifically, the commonly used preparation methods are as follows:
The pamine method: uses amines and acid chloride or anhydride to react under appropriate conditions to produce laxamide.
Non-classical acid catalytic methods: For example, after the medium in the catalytic reactor has been fully cured, the ferric chloride and acid catalyst can be converted to laclamide at low temperatures.
High-pressure reaction method: Laclamine is synthesized by imimine device and NBS in a high-pressure environment.
Safety Information:
Laxamide is a chemical and should be properly stored in a dry, cool place, away from fire sources and oxidants.
During operation, good ventilation conditions should be maintained and appropriate personal protective equipment such as protective gloves, face shields, and protective eyewear should be used.
Laclamide can be irritating to the skin and eyes.
When disposing of waste, it should be disposed of in an appropriate manner in accordance with local regulations.