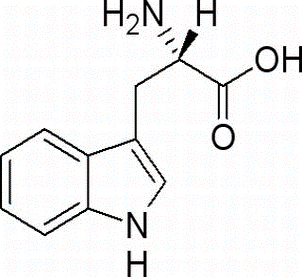
L-Tryptophan(CAS# 73-22-3)
| Hazard Symbols | Xi – Irritant |
| Risk Codes | R33 – Danger of cumulative effects R40 – Limited evidence of a carcinogenic effect R62 – Possible risk of impaired fertility R41 – Risk of serious damage to eyes R37/38 – Irritating to respiratory system and skin. R36/37/38 – Irritating to eyes, respiratory system and skin. R22 – Harmful if swallowed |
| Safety Description | S24/25 – Avoid contact with skin and eyes. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S36 – Wear suitable protective clothing. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| WGK Germany | 2 |
| RTECS | YN6130000 |
| FLUKA BRAND F CODES | 8 |
| TSCA | Yes |
| HS Code | 29339990 |
| Toxicity | LD508mmol / kg (rat, intraperitoneal injection). It is safe when used in food (FDA, §172.320, 2000). |
Introduction
L-Tryptophan is a chiral amino acid with an indole ring and an amino group in its structure. It is usually a white or yellowish crystalline powder that is slightly soluble in water and has increased solubility under acidic conditions. L-tryptophan is one of the essential amino acids that cannot be synthesized by the human body, is a component of proteins, and is also an indispensable raw material in the synthesis and metabolism of proteins.
There are two main ways to prepare L-tryptophan. One is extracted from natural sources, such as animal bones, dairy products, and plant seeds. The other is synthesized by biochemical synthesis methods, using microorganisms or genetic engineering technology for synthesis.
L-tryptophan is generally safe, but excessive intake may have some side effects. Excessive intake may cause gastrointestinal upset, nausea, vomiting, and other digestive reactions. For certain patients, such as those with rare hereditary tryptophan in the disease, ingestion of L-tryptophan may trigger more serious health problems.