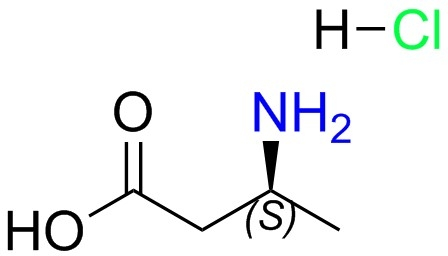
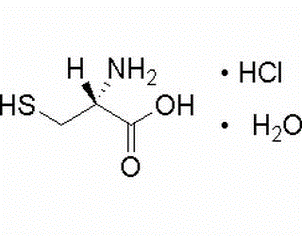
L-beta-homoalanine-HCl(CAS#58610-41-6)
| WGK Germany | 3 |
Introduction
S-3-aminobutyric acid hydrochloride, also known as S-3-aminobutyric acid chloride, is an organic compound. The following is an introduction to some of the properties, uses, preparation methods and safety information of this compound:- S-3-aminobutyric acid hydrochloride is a white crystalline solid that is soluble in water and polar solvents. – It has the hydrochloric acid properties of chloride and dissolves and releases chloride ions quickly. Uses:- S-3-aminobutyric acid hydrochloride is used more in chemical experiments and synthesis. – It is commonly used as a chlorination reagent, acid catalyst, chlorination reagent, etc. – It can also be used in chlorination reactions in organic synthesis, such as esterification, acylation, amino acid modification, etc. Preparation method:- S-3-aminobutyric acid hydrochloride is usually obtained by reacting S-3-aminobutyric acid with hydrogen chloride (hydrochloric acid). Safety Information:- S-3-aminobutyric acid hydrochloride has certain hazards to the human body, contact with skin and eyes should be avoided. – Appropriate personal protective equipment such as gloves, eyeglasses, and gowns should be worn when handling and handling the compound. – It should be used in a well-ventilated place to avoid inhaling its dust or vapors. – The compound needs to be protected from contact with strong oxidants, strong acids, and combustibles during storage and transportation.