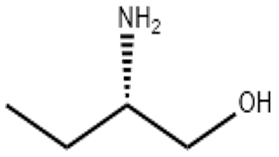
L-2-Aminobutanol(CAS# 5856-62-2)
| Hazard Symbols | C – Corrosive |
| Risk Codes | R34 – Causes burns R37 – Irritating to the respiratory system R22 – Harmful if swallowed |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) |
| UN IDs | UN 2735 8/PG 3 |
| WGK Germany | 3 |
| RTECS | EK9625000 |
| HS Code | 29221990 |
| Hazard Class | 8 |
| Packing Group | III |
Introduction
(S)-( )-2-Amino-1-butanol is an organic compound with the chemical formula C4H11NO. It is a chiral molecule with two enantiomers, of which (S)-( )-2-Amino-1-butanol is one.
(S)-( )-2-Amino-1-butanol is a colorless liquid with a pungent odor. It is soluble in water and common organic solvents such as alcohols and ethers.
An important use of this compound is as a chiral catalyst. It can be used in asymmetric catalysis in organic synthesis reactions, such as asymmetric synthesis of amines and synthesis of chiral heterocyclic compounds. It is also useful as an intermediate in drug synthesis.
The method for preparing (S)-( )-2-Amino-1-butanol includes two main routes. One is to obtain an aldehyde by carbonylation of a carboxylic acid or ester, which is then reacted with ammonia to obtain the desired product. The other is to obtain butanol by reacting hexanedione with refluxing magnesium in alcohol, and then to obtain the target product through reduction reaction.
Some safety precautions need to be paid attention to when using and storing (S)-( )-2-Amino-1-butanol. It is a flammable liquid and needs to be kept away from open flames and high temperatures. Appropriate protective equipment, such as chemical gloves and goggles, is required for use. Avoid contact with skin and inhalation of its vapors. Disposal is required in accordance with local waste disposal regulations.