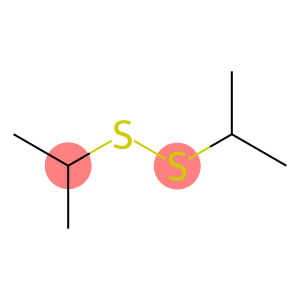
Isopropyl Disulfide(CAS#4253-89-8)
| Risk Codes | R11 – Highly Flammable R36/37/38 – Irritating to eyes, respiratory system and skin. R52 – Harmful to aquatic organisms R50 – Very Toxic to aquatic organisms |
| Safety Description | S9 – Keep container in a well-ventilated place. S16 – Keep away from sources of ignition. S29 – Do not empty into drains. S33 – Take precautionary measures against static discharges. S36 – Wear suitable protective clothing. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. |
| UN IDs | UN 1993 3/PG 2 |
| WGK Germany | 3 |
| HS Code | 29309090 |
| Hazard Class | 3.1 |
| Packing Group | II |
Introduction
Isopropyl disulfide is an organic compound. The following is an introduction to its nature, use, preparation method and safety information:
1. Nature:
- Isopropyl disulfide is a colorless to pale yellow liquid with a strong pungent odor.
- It is soluble in most organic solvents such as ethanol, ether, and benzene.
- At room temperature, isopropyl disulfide reacts with oxygen in the air to form sulfur monoxide and sulfur dioxide.
2. Usage:
- Isopropyl disulfide is mainly used as a reagent in organic synthesis and can be used in the synthesis of organosulfur compounds, mercaptans, and phosphodiesters.
- It is also used as an additive in coatings, rubbers, plastics, and inks to improve the performance of products.
3. Method:
Isopropyl disulfide is usually synthesized by:
- Reaction 1: Carbon disulfide reacts with isopropanol in the presence of a catalyst to form isopropyl disulfide.
- Reaction 2: Octanol reacts with sulfur to form thiosulfate, and then reacts with isopropanol to form isopropyl disulfide.
4. Safety Information:
- Isopropyl disulfide is irritating and may cause irritation and burns in contact with the skin and eyes.
- Avoid inhaling the vapor of isopropyl disulfide during use and avoid direct contact with the skin.
- Wear appropriate protective equipment, including gloves, goggles, and protective clothing, when using it.
- Seek medical attention immediately if inhaled or ingested.