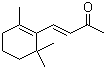
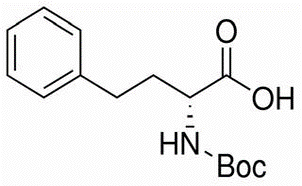
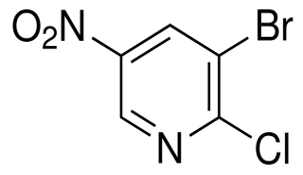
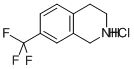
Irisone(CAS#14901-07-6)
| Risk Codes | R42/43 – May cause sensitization by inhalation and skin contact. |
| Safety Description | S24/25 – Avoid contact with skin and eyes. |
| WGK Germany | 2 |
| RTECS | EN0525000 |
| TSCA | Yes |
| HS Code | 29142300 |
introduce
nature
Violet ketone, also known as linaylketone, is a natural ketone compound. It is the main component of the aroma of violet flowers.
Violet ketone is a colorless to pale yellow oily liquid that is volatile at room temperature.
Violet ketone is soluble in alcohol and ether solvents, and slightly soluble in water. Its density is relatively low, with a density of 0.87 g/cm ³. It is sensitive to light and can absorb ultraviolet rays.
Violet ketone can be oxidized to ketone alcohols or acids in chemical reactions, and can be reduced to alcohols through hydrogenation reduction reactions. It can undergo alkylation and esterification reactions with many compounds.
Application and synthesis method
Violet ketone (also known as purple ketone) is an aromatic ketone compound. It has special fragrance and is often used in perfume and perfume industry. The following is an introduction to the uses and synthesis methods of ionone:
Purpose:
Perfume and spice: the fragrance characteristics of ionone, which is widely used in perfume and spice industry to manufacture violet fragrance products.
Synthesis method:
The synthesis of ionone is generally achieved through the following two methods:
Oxidation of Nucleobenzene: Nucleobenzene (a benzene ring with a methyl substituent) is subjected to an oxidation reaction, such as using an oxidizing acid or an acidic potassium permanganate solution, to generate ionone.
Coupling of Pyrylbenzaldehyde: Pyrylbenzaldehyde (such as benzaldehyde with pyridine ring substituents in the para or meta position) is reacted with acetic anhydride and other reactants under alkaline conditions to form ionone.