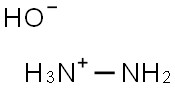Hydrazinium hydroxide solution(CAS#10217-52-4)
| Hazard Symbols | T – ToxicN – Dangerous for the environment |
| Risk Codes | R23/24/25 – Toxic by inhalation, in contact with skin and if swallowed. R34 – Causes burns R43 – May cause sensitization by skin contact R45 – May cause cancer R50/53 – Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. |
| Safety Description | S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S53 – Avoid exposure – obtain special instructions before use. S60 – This material and its container must be disposed of as hazardous waste. S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. |
| UN IDs | UN 2030 |
Hydrazinium hydroxide solution(CAS#10217-52-4)
quality
Hydrazine hydrate is a colorless, transparent, oily liquid with a light ammonia odor. In industry, the content of 40%~80% hydrazine hydrate aqueous solution or hydrazine salt is generally used. Relative density 1. 03 (21℃); Melting point – 40 °C; Boiling point 118.5 °c. Surface tension (25°C) 74.OmN/m, refractive index 1. 4284, heat of generation – 242. 7lkj/mol, flash point (open cup) 72.8 °C. Hydrazine hydrate is strongly alkaline and hygroscopic. hydrazine hydrate liquid exists in the form of dimer, miscible with water and ethanol, insoluble in ether and chloroform; It can erode glass, rubber, leather, cork, etc., and decompose into Nz, NH3 and Hz at high temperatures; Hydrazine hydrate is highly reducible, reacts violently with halogens, HN03, KMn04, etc., and can absorb C02 in the air and produce smoke.
Method
Sodium hypochlorite and sodium hydroxide are mixed into a solution in a certain proportion, urea and a small amount of potassium permanganate are added while stirring, and the oxidation reaction is carried out directly by steam heating to 103~104 °C. The reaction solution is distilled, fractionated, and vacuum concentrated to obtain 40% hydrazine, and then distilled by caustic soda dehydration and reduced pressure distillation to obtain 80% hydrazine. Or use ammonia and sodium hypochlorite as raw materials. 0.1% bone glue was added to ammonia to inhibit the transitional decomposition of hydrazine. Sodium hypochlorite is added to ammonia water, and the oxidation reaction is carried out under strong stirring under atmospheric or high pressure to form chloramine, and the reaction continues to form hydrazine. The reaction solution is distilled to recover ammonia, and then sodium chloride and sodium hydroxide are removed by positive distillation, and the evaporation gas is condensed into low-concentration hydrazine, and then different concentrations of hydrazine hydrate are prepared by fractionation.
use
It can be used as a glue breaking agent for oil well fracturing fluids. As an important fine chemical raw material, hydrazine hydrate is mainly used for the synthesis of AC, TSH and other foaming agents; It is also used as a cleaning agent for deoxidation and carbon dioxide removal of boilers and reactors; used in the pharmaceutical industry to produce anti-tuberculosis and anti-diabetic drugs; In the pesticide industry, it is used in the production of herbicides, plant growth blenders and fungicides, insecticides, rodenticides; In addition, it can be used in the production of rocket fuel, diazo fuel, rubber additives, etc. In recent years, the application field of hydrazine hydrate has been expanding.
security
It is highly toxic, strongly erodes the skin and blocks enzymes in the body. In acute poisoning, the central nervous system can be damaged, and in most cases it can be fatal. In the body, it mainly affects the metabolic function of carbohydrates and fats. Has hemolytic properties. Its vapors can erode mucous membranes and cause dizziness; Irritates the eyes, making them red, swollen and suppurated. Damage to the liver, lowering blood sugar, dehydration of the blood, and causing anemia. The maximum allowable concentration of hydrazine in air is 0. Img/m3。 Staff should take full protection, rinse directly with plenty of water after skin and eyes come into contact with hydrazine, and ask a doctor for examination and treatment. The work area must be adequately ventilated and the concentration of hydrazine in the environment of the production area must be frequently monitored with appropriate instruments. It should be stored in a cool, ventilated and dry warehouse, with a storage temperature below 40 °C, and protected from sunlight. Keep away from fire and oxidants. In case of fire, it can be extinguished with water, carbon dioxide, foam, dry powder, sand, etc.