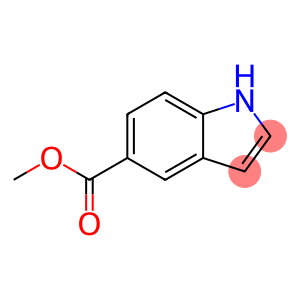
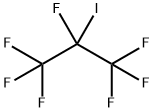
Heptafluoroisopropyl iodide(CAS# 677-69-0)
| Risk Codes | R20 – Harmful by inhalation R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S37/39 – Wear suitable gloves and eye/face protection |
| UN IDs | 2810 |
| WGK Germany | 3 |
| RTECS | TZ3925000 |
| FLUKA BRAND F CODES | 8 |
| TSCA | T |
| HS Code | 29037800 |
| Hazard Note | Irritant |
| Hazard Class | 6.1(b) |
| Packing Group | III |
Introduction
Heptafluoroisopropyliodine, also known as iodine tetrafluoroisopropane, is a colorless liquid substance. The following is an introduction to the properties, uses, preparation methods and safety information of isopropyliodine heptafluoroide:
Quality:
- Appearance: colorless liquid with a special odor.
- Stability: Heptafluoroisopropyliodine is relatively stable to light, heat, oxygen and humidity.
Use:
- Heptafluoroisopropyliodine is mainly used as a cleaning agent in the electronics industry. It has good cleaning performance and can effectively remove dirt and residue from the surface of electronic components.
- Heptafluoroisopropyliodine is also used in the semiconductor industry as a solvent for cleaning and etching in chip manufacturing, as well as as a film remover for photoresists.
Method:
- The preparation of isopropyliodine heptafluoroisopropyliodine can be obtained by the reaction of isopropyl iodide, magnesium fluoride, and iodine.
Safety Information:
- Heptafluoroisopropyliodine is highly irritating and toxic and should be avoided in contact with the skin, eyes, or inhalation. Protective eyewear, gloves and respiratory protection must be worn.
- When using heptafluoroisopropyliodine, ensure that the room is well ventilated and avoid contact with fire sources and high temperature environments to avoid explosions or fires.


![2-(Chloromethyl)[1,3]oxazolo[4,5-b]pyridine(CAS#110704-34-2)](https://www.xinchem.com/uploads/oxazolopyridine.png)
![yclohexene 1-[2-(triethylsilyl)ethynyl]-(CAS# 21692-54-6)](https://www.xinchem.com/uploads/yclohexene12triethylsilylethynyl.png)