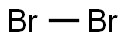Government regulation(CAS#7726-95-6)
| Hazard Symbols | T+ – Very toxicC – CorrosiveN – Dangerous for the environment |
| Risk Codes | R26 – Very Toxic by inhalation R35 – Causes severe burns R50 – Very Toxic to aquatic organisms |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. |
| UN IDs | UN 1744 |
Government regulation(CAS#7726-95-6)
Uses
Open Data Unverified Data
The main uses of bromine include:
- Disinfectant: Bromine is an effective disinfectant that can be used in fields such as water treatment and swimming pool disinfection. Unlike chlorine, bromine does not produce irritating odors and tastes, making it more friendly to the human body.
- Medical field: Bromine also has extensive applications in the medical field. Bromine compounds can be used to treat diseases such as epilepsy, asthma, and arrhythmia. In addition, bromine can also be used as a raw material for manufacturing some drugs, such as potassium bromide, ammonium bromide, etc.
- Food additive: Bromine can be used as a food additive, mainly in bread, dough, noodles and other noodle products. Bromine can increase the elasticity and ductility of flour products, making them softer. In addition, bromine can also be used in the production of beer, beverages, etc.
- Production of Bromides: Bromine is mainly used to produce bromides. As an important chemical raw material, it is widely used in various industries, including flame retardants, fire extinguishing agents, refrigerants, photosensitive materials, pharmaceuticals, pesticides, oil fields, and other industries. It can be used for the replacement of intermediates in raw materials such as dyes, pharmaceuticals, pesticides, etc. It can also be used as a regular analytical reagent, oxidant, absorbent for ethylene and heavy hydrocarbons, and as a brominating agent for organic synthesis.
Write your message here and send it to us