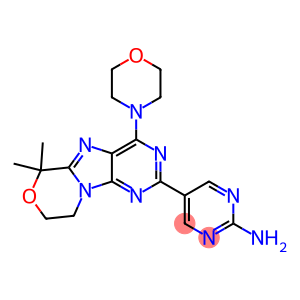
GDC-0084(CAS#1382979-44-3)
GDC-0084(CAS#1382979-44-3)
Mechanism of action: It is a small molecule inhibitor of the PI3K, AKT, and mTOR pathways, thereby inhibiting tumor proliferation by targeting key growth signaling pathways in cancer cells.
Clinical use: Mainly used in patients with glioblastoma with newly diagnosed, non-methylated MGMT who have completed initial radiation therapy with temozolomide. In August 2020, the U.S. Food and Drug Administration (FDA) granted fast-track designation to paxalisib for the treatment of these patients. Based on the results of the Phase 2 study, paxalisib has positive results in terms of safety and efficacy as a first-line treatment for patients with newly diagnosed glioblastoma. In the intention-to-treat population, patients treated with paxalisib had better median overall survival and median progression-free survival than those treated with temozolomide (historical data).
Adverse reactions: including hyperglycemia, oral mucositis and rash.
GDC-0084 has shown some potential in the treatment of glioblastoma, but more clinical trials are still needed to further validate its efficacy and safety.