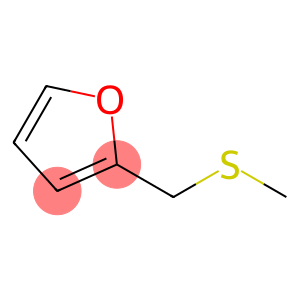
Furfuryl methyl sulfide(CAS#1438-91-1)
| Hazard Symbols | Xi – Irritant |
| Risk Codes | 36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S37/39 – Wear suitable gloves and eye/face protection S37 – Wear suitable gloves. |
| UN IDs | UN 3334 |
| WGK Germany | 3 |
| TSCA | Yes |
| HS Code | 29321900 |
Introduction
Methyl furfuryl sulfide, also known as methyl sulfide or thiomethyl ether, is an organic compound.
Chemical properties: Methyl furfuryl sulfide is a reducing agent that can react with oxygen or halogens. It can also undergo nucleophilic addition reactions with compounds such as aldehydes, ketones, etc.
The main uses of methylfurfuryl sulfide include:
As a solvent: Methyl furfuryl sulfide can be used as a solvent in organic synthesis reactions to promote chemical reactions.
Photosensitizer: Methyl furfuryl sulfide can also be used as a photosensitizer, which has applications in photosensitive materials, photography and printing.
The preparation method of methyl furfuryl sulfide is generally obtained by two methods:
Direct synthesis method: obtained by the reaction of methyl mercaptan and methyl chloride.
Displacement reaction method: obtained by reacting thioether with alkaline alcohol, and then reacting with methyl chloride.
Methylfurfuryl sulfide is irritating and may cause irritation to the eyes and skin, and protective equipment should be worn during handling to avoid contact with the skin and eyes.
When storing and using methyl furfuryl sulfide, avoid contact with strong oxidizing agents such as oxygen and halogens or flammable substances to prevent dangerous reactions.
Avoid inhaling the vapours of methylfurfuryl sulfide and work in a well-ventilated area with appropriate respiratory protection.
Do not discharge methylfurfuryl sulfide into water sources or drains to avoid polluting the environment.